![]()
<< To Homepage >>
<<Archives>>
December 2011 Archives:
December 21, 2011 -- 2:45pm EST Boston Scientific's New Drug-Eluting Stent in the News Again
I first heard concerns about stent deformation, primarily seen in the PROMUS Element stent made by Boston Scientific (NYSE: BSX), during a presentation at a small interventional meeting last summer. The issue was then reported in two journal articles, just prior to the TCT meeting in November, where it became a topic of much attention -- although most interventional cardiologists felt that it was nothing like the problem of late stent thrombosis, first seen as a "rare" event in the first generation of drug-eluting stents five years ago. I reported extensively on longitudinal stent deformation a few weeks ago when Dr. John Ormiston's article in JACC Interventions was published. Angioplasty.Org's coverage can be read in the following articles:
The Reuters article cites the thinner struts of the new generation of stents as a possible cause. However, it's not necessarily the thinness of the stent struts but the design and structure of the stent lattice that may be a reason for this vulnerability. For example, the photo at the top of this post compares Boston Scientific's Element stent design with Medtronic's new Integrity platform. Which one do you think would be more prone to the so-called "concertina effect"? The Element, in which the peaks and valleys line up and look, well, "squeezable" -- or the Integrity, where the peaks oppose each other?
This issue of stent compression was measured in Dr. Ormiston's study in which he bench-tested seven stents, confirming anecdotal reports that Boston's Element stent platform was more prone to stent compression (only four of the total are shown in this photo). But it's not just thinness because Abbott's XIENCE stent platforms (both the older XIENCE V and newer XIENCE Prime) both fared well, even though they, like the Integrity, boast thinner (and therefore more flexible) struts. And it gets more complicated because another factor cited in Ormiston's study was the number of connectors between the strut hoops. Element uses only two, as does Medtronic's decade-old Driver platform, which also showed a vulnerability to compression, although less than the Element. Abbott's XIENCE has three and the older CYPHER (the least compressible) has six. So do more connectors equal less compression? Sounds correct, except that Medtronic's Integrity, which performed very well, has only two connectors. Ormiston posited that this may be due to Integrity's unique design: it's fashioned from a single wire (hence the name, Integrity). So, no news here: the issue of stent performance is a complex engineering design calculation. What is meaningful, as the Reuters article points out, is that Boston's brand-new design is the basis for its entire product line of new stents. The article quotes Dr. Mark Ricciardi, director of Interventional Cardiology at the University of New Mexico Health Science Center, as saying:
If many interventional cardiologists feels the same way, Boston Scientific's stent division may have some problems, certainly against Abbott's XIENCE, and even more when Medtronic's new Resolute drug-eluting stent receives FDA approval, anticipated sometime next year. Speaking of the FDA, the "news" that prompted the Reuters article was that, when the FDA approved the PROMUS Element last month, it required Boston Scientific to include a caution in its labeling. As previously reported on Angioplasty.Org, the package insert for the PROMUS Element stent devotes Section 6.3 (page 4) to this issue, and the section leads off with this warning:
Dr. Cindy Grines of Detroit Medical Center and editor of the Journal of Interventional Cardiology and author of one of the first reports of the issue of stent compression, is also quoted in the Reuters piece:
Again, longitudinal stent deformation has been reported only in a small percentage of procedures. And, if recognized, it usually can be repaired on the spot. But we would guess that the executives at Boston Scientific are definitely keeping an eye on whether the number of these reports increases, as a result of "reading the label". « permalink » « send comment » « back to top »
December 7, 2011 -- 2:15pm EST A Stent By Any Other Name
The official "Appropriate Use Guidelines" place stent and angioplasty procedures into three categories: Appropriate, Uncertain and Inappropriate. Any patient, potential patient or, for that matter, anyone not steeped in the minutiae of interventional cardiology, would look at those terms and assume that any doctor putting a metal coil into someone's heart when the procedure was labeled "uncertain" or "inappropriate" should be fined or fired or both. Except they would be wrong. These terms specifically relate to the "level of evidence" in the official guidelines, evidence that has been adjudicated by a large committee that looked at clinical trials and studies and came up with categories and subcategories like Ia (the highest) and IIIb or IIc, etc. etc. But, if you are a patient and your particular clinical situation was not included in a large randomized clinical trial (and many are not), then your doctor's decision to treat would most likely fall into the "uncertain" or possibly the "inappropriate" category, even though his or her best medical judgement is that a procedure would benefit you. In other words these terms relate only to how they have been tested in large clinical trials -- i.e. there is clear evidence supporting the use of this particular procedure in this particular situation. Unfortunately, many clinical trials exclude large numbers of patients, often those who are the sickest, specifically because their inclusion will "muddy the waters" so to speak and confound any clear scientific results. For example, the oft-touted COURAGE trial, the one that showed no additional benefit for stenting over optimal medical therapy in stable patients, excluded 90% of the patients who were screened, because they did not fit the study's definition of stable angina. Yes, that's 90%! Only one out of ten patients screened for COURAGE was included in the actual study. I'm not saying that categorizing procedures in terms of levels of evidence is wrong -- there have to be definitions against which medical practice can measure and judge itself. But it's just that guidelines, as I've written before, are NOT rules or laws. They're just there to "guide" judgement. If you are a cardiologist and you have a patient whose condition falls outside the guidelines' medians, then you have to exercise your best clinical decision-making -- yet the procedure you perform, if it is labeled by some type of panel, might be called "uncertain" or "inappropriate". This does not mean "wrong" or "dangerous" or "harmful". It just means there's equivocal or no evidence in published studies for this situation. As FaceBookers would say: "It's Complicated" -- and it is -- for more on these issues regarding Approrpiateness Criteria, read my interview with Dr. Paul S. Chan. But back to my point about words. Cardiologists may have ongoing debates about these issues in journals, at national meetings, etc. but when guidelines or reports on "Appropriate Use of PCI" are issued, these reports are picked up by the public, the media and the evening news, and the precise words and labels used become important, and their intent changes from the strict narrow scientific definitions to broad-based meanings with all sorts of implied understanding (or mis-understanding) among the general population...and these labels usually appear in the headlines (or dreadlines, as I am wont to call them). A prime example are the following dreadlines that appeared right after the July publication of Paul Chan's study in JAMA, which showed that only 4% of PCI procedures were inappropriate:
So my recommendation is that a committee be organized to rework the terminology used in the guidelines -- a kind of guidelines for the guidelines committee. And this committee should be tasked with the goal of creating better labels and ways to communicate the safety and efficacy of these procedures to the public, as well as to the profession, payers and politicians. And, I would highly recommend that such a committee consist not just of doctors and scientists, but of members who are, in effect, wordsmiths: writers, journalists...perhaps even bards.... « permalink » « send comment » « back to top »
December 4, 2011 -- 5:05pm EST Stents, Angioplasty and PCI: The Uncertainty Principle (MEDICARE Style)
The bottom line is that, on November 15, CMS announced "New Demonstrations to Help Curb Improper Medicare, Medicaid Payments". These so-called "demonstrations" will occur in 11 states where claims "historically result in high rates of improper payments": Florida, California, Michigan, Texas, New York, Louisiana, Illinois, Pennsylvania, Ohio, North Carolina and Missouri. You can read their press release and not quite realize what this means, so here's the deal: according to preliminary publications, stenting and angioplasty in non-emergency cases are on the list for audits. CMS will review these cases and, only after the review, will CMS reimburse the providers. For cardiologists, this means that in these 11 states, if they perform a PCI, they and their hospitals will have to wait 30-60 days for a review of their decision -- and, if CMS decides they were wrong, and the stent procedure was "inappropriate", neither they nor their hospitals will be reimbursed. In fact, the hospital will wind up out-of-pocket for the entire incident, overnight stay, etc. But don't worry -- it's just a "demo project" of only 11 states.... A very interesting "demo" indeed, since these 11 states constitute 54% of the entire U.S. population!! Perhaps "demo" stands for "demolition"! Actually it's more like a "time-bomb". This announcement was made just as both the TCT and AHA annual meetings ended and the Veith vascular surgery symposium was just beginning in NYC. Most interventionalists were just getting their bearings back (am I in San Francisco or Orlando or NY and, oh yeah, how many people are coming to Thanksgiving?) and so the press release from CMS didn't get much play -- until the implications were revealed two weeks later in an analyst's letter and a Bloomberg News report on Friday in the San Francisco Chronicle, titled "Hospitals Tumble on Medicare Order for Heart Procedure Audit". And tumble they did. Tenet Healthcare plunged 11%, and heart device manufacturer Medtronic was down 6%; Boston Scientific, Volcano and St. Jude all dropped 7%. Yesterday, Shelley Wood's article in theheart.org detailed the issue for the cardiology community. But the big big question that I have, the elephant in the cath lab, so to speak, is "what is an inappropriate procedure?" and "who makes that decision?" Welcome to the "Uncertainty Principle". The issue of appropriateness of procedures has always been one of our prime concerns on Angioplasty.Org. Back in July, we posted several interviews and articles concerning the subject of "Appropriateness" for PCI (angioplasty and stenting) including an exclusive interview with Dr. Paul S. Chan, author of an extensive study of over a half-million patients that had just been published in JAMA. Appropriateness is a topic that has always followed the field of interventional cardiology. The level of attention usually peaks with certain events, such as the COURAGE trial, which questioned the use of PCI in stable patients, or with the allegations against Dr. Mark Midei for "unnecessary stenting." Paul Chan's review of a half-million PCIs from the NCDR database added a significant amount of data to this debate, but unfortunately the terminology that is used can be very confusing (the terminology comes from the Guidelines, not Chan's study). The categories are "appropriate", "uncertain" and "inappropriate". Here's how Dr. Chan explained them to me:
As for what is appropriate, there's a debate as to whether a 50% narrowing is a reason to do angioplasty; some say 70%. And, of course, these measurements are taken from standard angiography. Many cardiologists feel that angiography alone is not enough and that intravascular measurements, like intravascular ultrasound (IVUS) and fractional flow reserve (FFR) are really necessary to make this judgement. We're also not sure what the massive problem is, because Chan's study showed that only 12% of non-emergency PCIs were "inappropriate" (virtually none of the emergency angioplasties were). So will CMS be equating "uncertain" with "inappropriate" -- because that would be, according to Dr. Chan, a misinterpretation. Or, as I like to think of it, "uncertainty about the uncertain" -- it has a much more quantum physics feel to it. But "uncertain" is definitely not the same as inappropriate. It just means that, in this particular situation, the patient's clinical situation is not addressed or covered by the various published studies and trials and that there is no consensus about treatment. It does not mean the doctor's decision to do the procedure was incorrect. But back to the crux of the issue: who are these CMS people who will be auditing the decisions of cardiologists? And what criteria will they use? I mean there's a significant level of debate about this issue within the interventional cardiology community as it stands. Go to any interventional meeting and listen to the panel discussions. These are not a bunch of thieves trying to figure out how to gyp the government. These are respected scientists trying to digest and discern the vast amount of data generated by the hundreds of studies and presentations on display. Yes, there are guidelines, but guidelines are NOT rules or laws. They address average populations, they give guidance to physicians (hence the name). And yes, there are definitely outliers...and liars. There are a very few physicians who are out to defraud, but they do exist -- one Maryland doc was recently sentenced to 8 years in prison for unnecessary stenting. Again, regarding these audits, what measures will be used? An angiogram? A percentage stenosis? What about using FFR to determine if a narrowing is causing ischemia. The FAME study showed that stenting is best done when the FFR is .80 or lower -- but FFR is not fully reimbursed in the U.S. so we have a conundrum whereby CMS may not reimburse a stent procedure that they determine is inappropriate, but the very test that might answer the question of appropriateness is also not reimbursed. You could call it a "no-win" situation; I call it a Cath-22! You can read more about the FFR issue in our article, "FFR: Why Isn't Everyone Using It?" which brings me to another question: why would both Volcano and St. Jude's stocks fall so much on this news? Both companies manufacture FFR catheters which, one would think, should be a boom industry in a field where decision-making is so desperately looking for a threshold number to put down in the patient's chart and prove to CMS that, yes, this procedure was "appropriate"! Go figure -- and by all means, send me your comments! « permalink » « send comment » « back to top »
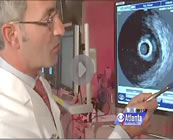
December 2, 2011 -- 7:30pm EST Women's Heart Disease and Intravascular Ultrasound (IVUS)
Now a similar tale has been broadcast by CBS affiliate WGCL-TV in Atlanta about how a type of coronary narrowing more typical in women may not be seen on a standard angiogram because it's evenly distributed along the arterial wall or channel and doesn't appear as a "spike" or sudden narrowing -- yet it may be restricting the flow of blood to the heart just the same. Once again -- angiography alone is not enough to accurately diagnose coronary artery disease and guide its treatment. Dr. Habib Samady of Emory University Hospital in Atlanta, Georgia explains more in this story and video clip on the WGCL-TV web site, reported by Kara Pesavento. (Emory, by the way, was the nexus for coronary angioplasty in the U.S. -- it's where Andreas Gruentzig moved to from Switzerland 30 years ago and where he taught thousands of doctors how to perform his then controversial new procedure.) « permalink » « send comment » « back to top »
December 2, 2011 -- 5:55pm EST Stent as Concertina
This is an issue that was first raised a little over a month ago and it has been the subject of a number of news articles. It's been dubbed "the concertina effect" but its scientific name is "longitudinal stent compression" or "longitudinal stent distortion" and it's of concern because once a stent has been correctly sized and placed in just the right position to keep a blockage open...well, you don't want it moving or changing shape.
Yet this can occur during an angioplasty -- rarely, but more often in some particular stents than in others, according to a bench-test that was published this week in JACC Cardiovascular Interventions. For a complete report on this bench-test, read our article, "New Boston Scientific Stent Shows Significant Shortening Compared to Other 2nd Generation DES" and, if you are an interventional cardiologist, read our follow up piece, "Avoiding and Repairing Coronary Stent Distortion", which contains tips and tricks from Dr. John A. Ormiston, lead author of the JACC study. |