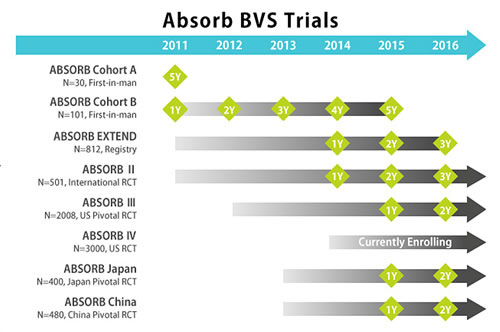
|
The Japanese approval was based on the results of three studies including AVJ-301 (ABSORB Japan), the ABSORB III clinical trial, as well as the ABSORB III Pharmacokinetic study. The manufacturer also provided a series of clinical trials, shown below. The primary end point was met in ABSORB Japan and ABSORB III demonstrating non-inferiority of BVS in comparison to Xience stent (DES).
Absorb GT 1 is indicated for symptomatic ischemic heart disease patients with newly developed coronary stenosis (lesion length less than 24mm) having a reference vessel ranging between 2.5mm and 3.75mm. Dr. Takashi Ouchi stated, “The first BVS product (Absorb GT 1) manufactured by Abbott Vascular was initially approved in Europe on April 30th in 2015 and the United States on July 5th in 2016. The major issue associated with Absorb GT 1 during the regulatory process was the inclusion of lesions smaller than reference vessel diameter (RVD) < 2.25mm in ABSORB III which resulted in higher TLF (cardiac death, TV-MI, and ID-TLR) (12.9% vs 8.3%) and scaffold thrombosis (ST) (4.62% vs 1.50%) rate in the BVS group than in the DES group.” Dr. Ouchi stressed that appropriate measurement of Reference Vessel Diameter (RVD), proper size of vascular scaffold, and relevant pressure of post dilatation were key elements for minimizing ST rate after implantation of BVS. Unlike the use of standard drug-eluting stents (DES), the operator must handle BVS more carefully. For example, delivering a post dilatation balloon catheter across BVS requires special caution which is not necessary during implantation of DES. According to the latest report demonstrating the clinical outcome of more than one year after the implantation, the number of clinical events (including ST) was considerably higher in the BVS group than in the DES group. PMDA carefully inspected its risk and benefit to make the final judgement of the product approval. The first and most important benefit regarding the BVS is its absorption property, whereas the risks include a higher incidence of ST and MI, clinical events during late and very late phase, and difficult/complicated procedure as compared to DES implantation. Although these issues remain unresolved, PMDA approved the clinical use of BVS under four conditions: 1) releasing appropriate guidance for use in cooperation with the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT); 2) collecting post market surveillance data; 3) collecting all ST events; and 4) reporting long-term outcomes included in ABSORB Japan study. Launch Schedule The number of investigator sites gradually will be increased after verification of lower ST rate (less than 0.9%) within the first three months of index procedure. Meanwhile, the investigator site policy will be modified if the 2-year ST rate of 250 cases of ABSORB Japan sites falls within 1.5%. If the rate of ST within the first 3 months is higher than 0.9%, investigations of the causes and supervision as to appropriate action to lower ST will be imposed. Participation in the product training program provided by the company (Abbott Vascular) is mandatory for the use of BVS in Japan. The first three cases must be performed under the surveillance of a well-trained interventional cardiologist with sufficient knowledge of the product or an appropriate representative of Abbott Vascular. Additionally, prospective investigator sites must have a vascular imaging system (IVUS or OCT) with the presence of an interventional cardiologist familiar with imaging guided intervention. A prospective investigator site is defined as a site enrolled in ABSORB Japan or a site with the presence of a CVIT certified interventional cardiologist performing more than 100 PCI cases per year. For additional requirements of further expansion of prospective investigator sites, a certified interventional cardiologist authorized by CVIT must be present and must agree to provide detailed information when ST occurs. Dr. Ouchi concluded, "BVS will be available in near future. We will accumulate data to drive the product in right direction." Source: This article was provided to Angioplasty.Org by its Japanese partner, TCROSS NEWS |