![]()
<< To Homepage >>
<<Archives>>
May-July 2008 Archives: July 26, 2008 -- 2:40pm EDT CT in the OC and the DC He has quotes from several OC sources, including Doug Ryan from CT manufacturer Toshiba (they're in Tustin, just down the freeway). But Stewart discusses the reality of the radiation situation and makes several good points, especially in light of all the negatively-skewed press (wag of finger) that CTA has recently gotten. We're also happy to see he's placed a link to our coverage as well (tip of hat). Across the continent in Bethesda (well, not precisely DC, but close enough) the National Heart, Lung and Blood Institute (NHLBI) of the NIH just concluded a two-day panel of "All-Stars", according to Shelley Wood of the theheart.org, to discuss the issues around cardiovascular imaging and diagnosis. Wood reports that the panel leaders, Drs. Allen Taylor and Michael Lauer, told her:
Perhaps this get-together represented a cooling-off period, because last fall Dr. Lauer was a very vocal naysayer at the annual AHA meeting and expressed strong anti-CT comments. According to this report, also from theheart.org, Lauer definitely squabbled, denouncing CTA and calling for a moratorium:
So it will most interesting to see what Dr. Lauer et al come up with after last week's NIH panel in the way of official requests for studies or clinical trials.
« permalink » « send comment » « back to top » July 12, 2008 -- 7:30pm EDT (updated) Passing of an Era -- Michael DeBakey (1908-2008) When producing the documentary, "Vascular Pioneers: Evolution of a Specialty", I was lucky enough to locate this two-hour interview, and even more lucky, to get permission to include parts of it in the program (which also included Bill Blaisdell). As I sat in my editing room, screening the 120 minutes, I was astounded at the detail and breadth of this man's experience. His stories about his mother's sewing classes (where he learned the technique that would revolutionize vascular surgery!) to his dinner with General Patton in Patton's French chateau during WWII, to his surgery on the Duke of Windsor who, like Arethra Franklin, refused to fly and took a train from NYC to Houston -- I was constantly reminded of what an amazing impact this one human had on the field of medicine. And I kept saying to myself, "This man was 87 years old when the interview was taped!! -- and he remembers more than most of us ever will experience in several lifetimes!" The era that is passing, referred to in the title, concerns the current and future treatment of aneurysms and other vascular disease, which is more and more being performed using an endovascular or minimally-invasive approach, using wires, balloons, stents and newer devices, without open surgery. This transition has been occurring over the past two decades -- trials are now ongoing in which heart valves are being replaced percutaneously, with a catheter through the arteries and into the chambers of the heart -- no open heart required. But none of this could have existed without the courageous and pioneering work of the first wave of modern surgeons -- and DeBakey was right in front. You can see a short clip from the interview with Dr. DeBakey, describing how he invented the synthetic Dacron graft, in our article about him .
« permalink » « send comment » « back to top » July 7, 2008 -- 7:45pm EDT CT Tour of the Heart "CT Heart Scan Experts Criticize New York Times Article" and the title says it all -- extensive quotes from no less than nine imaging specialists, refuting the one-sided presentation by the "paper of record" on an issue of great importance to patients and professionals alike. If you read the New York Times article, you should definitely read this.
« permalink » « send comment » « back to top » June 29, 2008 -- 1:45pm EDT Misleading Report on CT Heart Scans
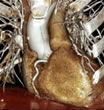
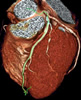
The headline is a point-of-view give-away: those money-grubbing docs, needlessly exposing their patients to dangerous radiation just to make a buck! (Odd that this article would appear in the midst of a giant political battle on Capitol Hill over big Medicare cuts for physicians.) It's a lengthy article and includes quotes from many sources. The authors, Alex Berenson and Reed Abelson clearly did a lot of legwork, but they vaulted over significant pieces of information that would definitely muddy up their thesis. Evidence-Based Medicine Radiation Risk CTA Will Lead to More Testing So how do these patients with no disease wind up on the cath lab table? Very often because of false positives from nuclear stress tests, often in women. Multislice CT Angiograms are 99% accurate in identifying patients with no coronary disease (see the CorE 64 study) so in many of these cases, both a nuclear stress test and invasive catheterization could have been avoided by using a single non-invasive less-risky test: the CT angiogram. Use of CTA is Indiscriminate But here's what we are not told. The patient was experiencing chest pain which, according to Dr. Hecht (I contacted him), was one of the major reasons for the CT scan, especially in light of the clear stress test. The New York Times never mentions this critical symptom -- not to its readers, and perhaps not to Dr. Brindis. One would have to wonder why?! Did not the authors' colleague, Tim Russert, who also had an elevated calcium score, pass his stress test with flying colors just a month before his fatal heart attack? His doctor told NBC that the autopsy showed "significant coronary artery disease in the Left Anterior Descending coronary artery." 15 minutes in a CT scanner would have revealed this -- the scan wouldn't have prevented the tragedy, but the elevated risk it indicated perhaps could have been treated more aggressively. Industry is Pushing CT Inappropriately I might point out that these four companies also manufacture and display every imaging device and modality used by all cardiologists, from full-blown catheterization labs (which take up the majority of their exhibit space) to X-ray units, MRI systems, ultrasound devices and yes, even nuclear stress units. They have very large booths, because all this equipment is...well...very large! Cowboys Of course any new technology can be overused, abused, applied incorrectly, etc. That's not what we're talking about here. We're talking about an important imaging technology that has been studied, discussed, compared, complete with Appropriateness Criteria guidelines issued jointly two years ago (!!) by all the professional cardiology and radiology societies. The leaders in this field, including the founding members of the Society of Cardiovascular Computed Tomography (SCCT), are very concerned that this technology is used appropriately and their concerns are reflected in the series of interviews we publish in the "Imaging and Diagnosis" section on Angioplasty.Org Dr. Hecht, in particular, was involved in the pioneering stages of coronary angioplasty, when he was assessing the results of balloon dilatation with nuclear scans. As he states at the close of his interview on Angioplasty.Org, "I have a huge background in both nuclear and stress echocardiography, and CTA is simply a better test. It's time to move to the better test." And now, back to my weekend.... « permalink » « send comment » « back to top » May 17, 2008 -- 10:50pm EDT Obama and Clinton Agree The article by Kevin Sack is a good read, and should be of significant interest to hospital administrators, physicians and patients. The "disarming approach" being put forward is that, if and when a medical mistake occurs, the doctor and hospital should immediately apologize to the patient, explain what happened and be forthcoming with all information instead of adopting the "deny and defend" strategy advocated by malpractice lawyers and insurers. To bolster the argument is the case study of the University of Michigan Health System which, according to Richard C. Boothman, the medical center’s chief risk officer, saw a very large drop in lawsuits, settlement amounts and time to settlement after instituting such a program. Boothman is quoted that "Improving patient safety and patient communication is more likely to cure the malpractice crisis than defensiveness and denial." So what does this have to do with Barack Obama and Hillary Clinton? Well, almost two years ago to the day, they co-authored an article in the New England Journal of Medicine titled, "Making Patient Safety the Centerpiece of Medical Liability Reform". Yes, co-authored! They agreed on something. And their 2006 article portrays precisely the program described in tomorrow's New York Times story. It even used the University of Michigan as the prime example where this has worked. In fact, the NEJM offers an audio interview with the same Richard Boothman as part of the article online. Back in May 2006, we featured the Clinton-Obama article on our news page because we thought it very forward of two highly regarded political leaders to promote such a carefully thought-out program in a peer-reviewed scholarly medical journal. It's quite likely in fact that the "prominent medical centers" now experimenting with this strategy read the NEJM piece when it was published. So how come the Times didn't acknowledge this forward-thinking journal article from two years ago? The Times piece does briefly mention that Clinton and Obama had sponsored some legislation in this area, but the Senators' NEJM article is not mentioned once in the NYT story, which seems a bit odd to me... « permalink » « send comment » « back to top » May 15, 2008 -- 10:50am EDT "Life Wide Open": A Stent Cypher (By the way, in case you're not familiar with the term "DTCA", the commonly-used acronym is actually "DTC" which stands for "Direct to Consumer", but hey, DTCA rhymes with PTCA, a.k.a. angioplasty -- so why quibble?) The NEJM "Perspective", was penned by two clinical cardiologists, Buffalo-based Dr. William E. Boden, principal investigator for last March's COURAGE trial (a.k.a. "Spring Awakening" for interventionalists) and Dr. George A. Diamond of Cedars-Sinai in L.A., co-author of one of my favorite analogy-genre pieces from October 2006 about the danger of stent thrombosis being greater than that from E. coli-laden spinach. Regardless of one's opinion of optimal medical therapy or delicious green vegetables, both Drs. Boden and Diamond have made it clear that they oppose stent-evangelism and have cautioned regularly against the overuse of interventional procedures. (For some short-term historical context on the tensions between clinical cardiologists and the stent-evangelists, read my post from last year, "Banned in Boston ".) The authors say the ad is "deceptive advertising" and find the idea of marketing a stent brand directly to patients an "experiment in interventional psychology". Cute. As for the so-called ad campaign, according to this morning's New York Times, it is no longer running except, for some inexplicable reason, in Baltimore. Something to do with over-"The Wire", no doubt. Or possibly the fact that the Baltimore TV market includes Rockville, Maryland where today and tomorrow the FDA Risk Communication Advisory Committee is holding hearings, specifically about DTC. In point of fact, according to the New England Journal, the hearings are the reason for the editorial being published online yesterday, in advance of print. But what's up here? Even Boden and Diamond told the NYT that, “the notion that television viewers inspired by such an ad would go to their physicians and request not only a stent but a specific brand and model of stent is frightening, if not utterly absurd.” Absurd, yes. It's one thing when a DTC-TV ad tells you about a new allergy med or sleep-aid and you go to your GP and mention it and he/she just happens to have samples of the pill left by a pharma detailer. Gimme! A stent -- slightly different. No free samples for one; and tens of thousands of dollars for a procedure (with associated risks) to put one in. So who really is the audience? Dr. Boden was more on target when he was quoted back in December by the Wall Street Journal Health Blog. He said, "You’ve got to wonder whether it’s a sign of desperation." Desperation, hmmm. Let's see. In March 2007, Dr. Boden's own COURAGE trial was presented at the American College of Cardiology with tremendous fanfare and backstage intrigue. The heads of both the ACC and AHA declared the study as "shaking the foundations of interventional cardiology" and that "hundreds of thousands of Americans with stable angina who received coronary stents did not need them". Coming upon the heels of concerns over late stent thrombosis, drug-eluting stent use dropped precipitously, from 90+% to the low 60's. Over a billion in sales was lost. I call it "free-DTC". Study presented, pronouncements made, newspapers run stories, patients see news, get worried, call doctors, and so on. No expensive TV spot necessary. Yet in their editorial, Drs. Boden and Diamond write:
However, just ask any interventional cardiologist (I did) and they'll tell you that's exactly what happened last year. Patients were actually requesting the good ol' tried and true bare metal stent. ("I'll take restenosis over thrombosis, doc!") You can read ample evidence of these patient preferences in the Forums on Angioplasty.Org, as well. But back to J&J's Thanksgiving desperation. It's November 2007. Not only is the U.S. DES market down overall, but the duopoly in these devices, shared by J&J and Boston Scientific, is rapidly coming to a close. The FDA Panel has just recommended Medtronic's new Endeavor stent and scheduled a review for Abbott's XIENCE. In fact, exactly one week after J&J's spot aired, the XIENCE was recommended for FDA approval (final approval is expected later this quarter). So who was this egregious TV ad really aimed at? If it was solely for patients, it wasn't a very good campaign. Standard advertising wisdom is that ads must be repeated often to be effective. But the spot didn't run often, certainly not enough to sway consumers. It reminded me of the full page ad that Boston Scientific took out in the New York Times, Wall Street Journal, Boston and Minneapolis papers, etc. the Monday after the FDA Stent Safety hearings in December 2006. It "answered every question that Frank Kemp had about drug-eluting stents". Again, it was a one-time ad and it's my opinion that it, along with J&J's TV spot, were aimed as much at the investment community, and the citizens of towns where these companies did business, as they were at patients. And they certainly were aimed at these companies' actual customers: the interventional cardiologists and their hospitals. ("See our ad? We're supporting you in this difficult time!") Really more like PR than advertising. DTC for devices is certainly a valid issue for the FDA panel to discuss, but I have a novel idea. What about using the vast dollars spent on these TV spots and full page ads to deliver a much more important message about stents and angioplasty "direct-to-consumers"? Like this one:
It's not controversial. It's not deceptive. Every study done on the subject agrees that angioplasty is the gold standard of care for acute MI. Heart attacks used to be fatal -- now an amazing number of lives are saved through the emergency use of angioplasty and stents. But unfortunately, not enough people are aware of this. Besides the big problem of denial of symptoms, many patients still don't understand that "time is muscle" and that opening up a blockage within a couple hours of symptoms can prevent damage to the heart. The hospital you go to can have a major effect on how you'll spend the rest of your life. And TV is a perfect medium to communicate this message. (Disclosure: I produced such a spot a decade ago for the San Francisco Heart Institute at Seton Medical Center.) I've railed before about the terrible portrayal of heart attack treatment on TV ("Don't Have a Heart Attack in Stars Hollow"). Maybe it's time for real DTC! By the way, a short note to J&J -- if you're going to create a branded ad campaign, start with a better name. Sure, "Life Wide Open" is a shout-out to what the stent does and how much better you're supposed to feel if your arteries (and lives) are "wide open". But rather than hearing this from stent-evangelists, we've now entered the territory of actual evangelists: namely "Life Wide Open", the title of a popular book by conservative evangelical radio/TV pastor David Jeremiah of the Turning Point Ministries. Also the name of a popular Knoxville-based Christian Rock Band. J&J's campaign may have a registered trademark™, but it's swamped by the competition in a Google search. At least spring for the 10-cents-a-pop GoogleAd to support the campaign. This same type of name choice was recently made by the American College of Cardiology with their new "patient site", CardioSmart. Sounds good, especially if you want 60 Softgels at the amazing price of $11.85!! |

 These
two photos of Michael DeBakey, taken half-a-century apart, give
a narrow sense of the wide span of his life. The first, taken (we
believe) in the 40's, shows a young surgeon, filled with possibilities
-- possibilities which, I might add, were mostly fulfilled. The
second was from an interview, conducted in December 1995 by F.
William Blaisdell, MD, FACS with Dr. DeBakey, who was Blaisdell's
mentor and teacher.
These
two photos of Michael DeBakey, taken half-a-century apart, give
a narrow sense of the wide span of his life. The first, taken (we
believe) in the 40's, shows a young surgeon, filled with possibilities
-- possibilities which, I might add, were mostly fulfilled. The
second was from an interview, conducted in December 1995 by F.
William Blaisdell, MD, FACS with Dr. DeBakey, who was Blaisdell's
mentor and teacher.