| << To Blog Home >> | |
|
|
May 27, 2010 -- 3:50pm EDT Stent Helpers: IVUS, OCT and FFR -- Intravascular
Tools At EuroPCR Briefly, OCT and IVUS are ways of imaging the inside of a coronary artery during a standard invasive catheterization or angioplasty. Both of these imaging technologies can help the cardiologist see things not visible on standard X-ray angiography (an angiogram is essentially a "shadow" image). The placement, sizing and expansion of a stent can all be measured more accurately via intravascular imaging. There is a difference between OCT and IVUS. OCT is a far higher resolution image, but IVUS penetrates the arterial wall deeper, revealing anatomical information hidden below the surface. All of this is discussed in our interview with OCT expert Dr. Giulio Guagliumi who discusses how both imaging modalities are useful and that the "combination is the true projection for the future". FFR measures intracoronary blood pressure and has been shown in the FAME study to be useful in decision-making about whether or not a blockage needs to be stented. In FAME, FFR-guided stenting used 1/3 less stents and improved outcomes by 28%. Recent news for OCT was that earlier this month the FDA approved LightLab's OCT device -- the first approval for this technology in the United States. Two weeks later, having seen a future for OCT, St. Jude Medical announced it was acquiring LightLab for $90 million. A competing OCT system from Volcano currently has European approval and is awaiting an okay from FDA; the company anticipates commercial release in the U.S. sometime mid-2011. Volcano has been a leader in IVUS imaging since its inception and at EuroPCR announced a new digital IVUS catheter, the lower profile Eagle Eye® Platinum, which is more deliverable and can fit through a 5F guide, making it ideal for transradial procedures done via the wrist. The only other IVUS system is the iLab, manufactured by Boston Scientific. An interesting new cross-over product was also introduced by Volcano, the VIBE™ RX Vascular Imaging Balloon Catheter, which combines IVUS with a dilatation balloon to provide image-guided therapy. This is a concept being explored by Volcano -- and a future system of Forward-Looking IVUS may make inroads into solving the problem of chronic total occlusions (CTO). Andreas Gruentzig, the inventor of coronary angioplasty, once told me that unless angioplasty could solve the problem of CTOs, it would never be able to completely compete with bypass surgery. Finally FFR was in the news as the two-year FAME data, first presented at TCT last September, were published online in JACC. The original findings were sustained, leading to the conclusion that:
This conclusion is one reason why 18 months ago St. Jude Medical (them again?) acquired the Swedish manufacturer of FFR catheters, Radi Medical Systems. The competing FFR system is made by...Volcano (them again?) and it just received FDA and CE Mark approval for its latest FFR product, the PrimeWire PRESTIGE™ Pressure Guide Wire. So, to recap the acquisitions and new product introductions: St. Jude Medical now offers FDA-approved OCT and FFR; Boston Scientific offers IVUS; Volcano offers IVUS, FFR and anticipates FDA approval of OCT -- the company is also poised to launch various new "image-guided therapies". (BTW, a report yesterday on theheart.org states that, with its recent acquisitions, "St Jude boasts the only intravascular ultrasound platform with both OCT and fractional-flow-reserve technology." To our knowledge, St. Jude does not offer an IVUS system, unless they bought Boston Scientific when we weren't looking.) One last point is that the advantage of utilizing products from the same manufacturer is being able to use the same console or the same integrated cath lab system with all devices, especially if they are used in combination, in order to minimize the extra time and effort required to add on these intravascular "stent helpers" for the benefit of patients and procedural outcomes. |
|


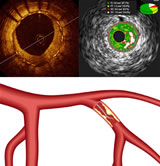 This
graphic shows three intravascular imaging/therapeutic modalities that
were making news this month, both in the U.S. and in Paris
at the EuroPCR meeting ending tomorrow. Clockwise
they are Optical Coherence Tomography
(OCT), Intravascular
Ultrasound
(IVUS)
and Fractional Flow Reserve (FFR). You can read more about these
in depth in Angioplasty.Org's
This
graphic shows three intravascular imaging/therapeutic modalities that
were making news this month, both in the U.S. and in Paris
at the EuroPCR meeting ending tomorrow. Clockwise
they are Optical Coherence Tomography
(OCT), Intravascular
Ultrasound
(IVUS)
and Fractional Flow Reserve (FFR). You can read more about these
in depth in Angioplasty.Org's