
Click
here for more information about the AWARE
clinical trial (Angiogenesis in Women With
Angina Pectoris Who Are Not Candidates for
Revascularization) |
|
Q:
Could you briefly go over the description of
what the AWARE trial is, what type of patient
you're looking for, and what the goal is?
Dr.
Moreadith: The AWARE trial is a Phase 3 trial
-- a pivotal stage trial - that's going to
be evaluating patients, women between the ages
of 18 and 75 who have Canadian Cardiovascular
Society (CCS) Class III or IV angina. They
are women who are not candidates for, or would
be unlikely to benefit from, a standard revascularization
procedure. Typically, that means these are
women who have undergone angioplasty and/or
bypass surgery; they're on maximal medical
therapy; and yet their life is still limited
by chronic angina. So this study will evaluate
and enroll them, in a randomized fashion, to
a placebo group or one of two dose groups,
and evaluate the effect of Ad5FGF-4, also known
as Generx, on exercise endpoints that indicate
ischemia. The
key primary end point in the study will be the
time to onset
of myocardial ischemia that is typically done with
a routine exercise treadmill test. |
The
key secondary end point will be SPECT perfusion,
a non-invasive
method that measures myocardial blood flow in the
region of the heart that is ischemic. What we hope
to find is that not only do women who receive the
drug experience prolongation of time to onset of
myocardial ischemia – that is – they
can walk longer on the test before developing ECG
changes indicating ischemia, but also a reduction
in that perfusion deficit as measured by SPECT
perfusion.
Q: You’re
delivering the Ad5FGF-4, or Generx, using
a virus. Tell
us about that.
Dr. Moreadith: Sure. This is a crippled virus
called Adenovirus Type 5. This is a very common
virus; most of the people in the United States
have had a mild flu-like syndrome, or sniffles,
and it's often caused by this type of virus.
We've genetically engineered this virus to
cripple it so it cannot reproduce, and it's
been shown to be safe following administration
into the coronary arteries. It carries a gene
known as fibroblast growth factor 4. We've
studied this in 450 patients who have received
an active dose of this via infusion into the
coronaries, and we've found no adverse events
associated with doing that. What we have found
is it's taken up almost exclusively by the
heart. So, it delivers the gene and appears
to stimulate the growth of new blood vessels
in the ischemic region of the heart.
Q: What is the
procedure -- just sort of a “step
one through five” – that participants
will undergo?
Dr. Moreadith: Patients will be randomized
at multiple centers in the United States. We
will be working closely with approximately
50 major medical centers in the United States,
and eventually enroll about 300 patients in
this study. They'll undergo routine screening
with an exercise test, and they'll have a SPECT
perfusion study done as well. In many cases,
the women who are candidates for this study
are used to these procedures, because they've
had these done at some point during the course
of their disease. If they are eligible for
the study, they'll be randomized, come into
the cath lab, and they'll undergo a controlled
infusion of this product down the coronary
arteries. Our aim is to put 60% of the dose
in the left side of the heart, in both native
vessels or bypass vessels, and 40% of the dose
in the right side of the heart. In this manner
we expose the coronary circulation in the heart
with the virus so that wherever there is ischemia
in the heart, hopefully this will stimulate
the growth of new blood vessels.
Q: The AWARE trial has already begun -- how
many centers do you have actually online at
this point?
Dr. Moreadith: We update that almost weekly
now, on our Clinicaltrials.gov
site.
Most of the sites have already been given approval
by their Internal Review Boards.
Q: What is the time frame right now? When
do you see this being completed, and how long
will you be following patients once they are
given the Generx?
Dr. Moreadith: The key primary end point, as
I mentioned, is time to onset of ST segment
changes diagnostic of myocardial ischemia.
That time point will be recorded at 6 months
after the dosing. At the same time point, we'll
measure the key secondary end point, which
is the adenosine SPECT perfusion study. It
kind of depends on how quickly we get patients
in the study. We certainly hope to have the
vast majority of these patients enrolled in
the study within the next two years, and then
secondary efficacy end points will go out to
one year past the last patient in the study.
Q: This isn’t the first clinical trial
you’ve conducted with Generx. You’ve
had previous experience with it.
Dr. Moreadith: Actually, we have the largest
safety and efficacy database of a DNA-based
cardiovascular therapeutic in the world. We've
studied 663 patients: 213 of those patients
received placebo, and 450 received an active
dose. That is the result of four angiogenic
gene therapy trials, AGENT-I, II, III and IV
-- and all of those were randomized, placebo-controlled,
double-blind studies at over 100 medical centers
worldwide. So, the safety and efficacy database
of those four programs is very extensive, and
we feel very comfortable advancing this to
Phase 3 development in women with refractory
angina. I might add that the FDA also feels
very comfortable with that, and they've highlighted
this program by giving it Fast Track designation
as addressing a large unmet clinical need.
Q: Can you expand
on that? This is a very important potential
therapy and, while there
have been studies in the past, it’s my
understanding that no one has gotten to this
phase of research previously.
Dr. Moreadith: That is correct. This is the
first time that a DNA-based cardiovascular
therapeutic has advanced to pivotal Phase 3
development. And, to my knowledge, this is
one of very few programs in cardiovascular
disease that has ever been given fast track
status by the FDA. That means that the FDA
recognizes this as a large unmet clinical need,
and will give this priority review once we
go back to the Agency with the data. So, it
is a precedent-setting trial: it's the first
Phase 3 study of its kind and it's one of the
very few cardiovascular interventional studies
conducted in women only.
This really represented
a sea change in the Agency’s thinking, as well as ours – the
recognition that this product produces a permanent
change in the heart. It's not a typical anti-anginal.
This product grows new blood vessels in the
heart and is thus a disease-modifying agent.
We were encouraged to explore a primary end
point that reflected that. So in discussions
with the FDA, we agreed that the time to ST
segment depression, indicative of myocardial
ischemia, was an adequate end point for that
effect, and we will pursue the indication of
myocardial ischemia in patients with refractory
angina.
Q: Why is this trial for women only?
Dr. Moreadith: That's a very good question.
The pooled analysis of our data from all
of the AGENT studies, in particular AGENT
III and IV, indicated that a large proportion
of the patients who were randomized in AGENT
III and IV were mostly men, and over half
of those patients had mild angina. So, because
of a profound placebo response, largely in
men with Class II angina, we were unable
to measure a true treatment response. However,
it was pre-specified in the analysis plan
that we would perform a gender analysis as
well, and when we did, we were surprised
to see a profound treatment effect in women.
What we found was a very low placebo response
rate in women, largely because these women
were sicker: they had more symptom-limited
disease, there was a higher percentage of
patients with CCS Class II and IV, and the
vast majority of these women were on triple
therapy as well. So, it appeared that in
female patients with more severe disease,
who were more symptom-limited and exercise-limited,
that the intra-coronary infusion of Ad5FGF-4
did in fact promote a profound treatment
response. In fact, on every clinical endpoint
in women we observed a concordance of treatment
effects – time to onset of ischemia,
total exercise duration, time to onset of
angina, and change in functional CCS class
out to one year. So, we took that data to
the FDA, and the Agency agreed with us that
we had sufficient evidence, in terms of both
safety and efficacy, to proceed to a Phase
3 trial.
Q: This is an interesting example of a trial
that looked like it had not succeeded, had
not produced results, but when you looked at
the data by gender, you discovered that in
fact it did work in women.
Dr. Moreadith: Yes. But I want to point out
- we don't think this drug works only in women.
It appears to work in women with this particular
end point – time to changes diagnostic
of myocardial ischemia, but in our AGENT-II
study, which was largely men, we found a very
significant reversal in perfusion deficit size
by SPECT. So, even in men who have a perfusion
deficit, who may not show a change in their
exercise treadmill times, there was a reversal
in the perfusion deficit. So, the AWARE study
is designed with SPECT as a key secondary end
point, and in the future, we hope to be able
to take this data back to the Agency and perhaps
use SPECT as a surrogate end point in both
men and women.
Q: An interesting piece of data from the controversial
COURAGE trial, one that's not talked about
very much, is that 25% of the patients studied,
whether they had angioplasty or medical therapy,
were still experiencing angina after a five-year
period. Does that have relevance to the trial
you're working on?
Dr. Moreadith: The COURAGE trial was a precedent-setting
study in many respects and I think what it
underscores is that the treatment of patients
with chronic angina represents a very large
and growing population of patients. Despite
what we consider to be optimal medical therapy
in many of these patients, including stable
medical regimens as well as interventional
approaches, while it may diminish the risk
of death, or myocardial infarction, or re-hospitalization
acutely, it does not really change the natural
history of the disease. So, these patients
are forced to live with chronic angina. And
I think that we'll find in the next decade
that, as we continue to improve at putting
stents in and doing bypass surgery, we'll need
another approach to treat chronic stable angina
in these patients, because it really does severely
limit their lifestyle.
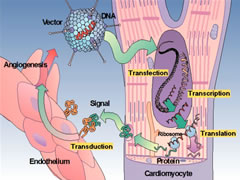
The
Four "T"s of adenovector gene therapy.
(Click to enlarge) |
|
Q:
The mechanism by which this works is not
an unnatural one to the body-- my understanding
is that you're kind of inducing the body
to do what it would normally do in many
cases?
Dr. Moreadith: That's exactly right. In fact, in many respects, it seems that
this therapy is uniquely designed at this point in adulthood to stimulate the
growth of new vessels. FGF4, the ligand for the FGF4 receptor, is the gene that
we express in the myocardium from the virus. It turns out that the adult heart
has plenty of FGF4 receptors that would bind the ligand. But soon after embryogenesis
FGF4 levels drop to zero throughout the adult body. It appears FGF4 is very important
in laying down the embryonic vascular bed, but soon thereafter FGF4 is no longer
expressed. Yet, the receptor stays at very high levels in the heart throughout
adulthood. |
And so, we deliver
the ligand through the Ad5FGF-4 adenovector,
and it binds to the FGF4
receptor that's at high levels of expression
in the heart. So, we “re-trigger” the
growth of blood vessels within the myocardium.
We're simply taking advantage of a biological
architecture within the body that is designed
to grow new blood vessels if the ligand is
present. About 90% of the adenovector stays
within the myocardium, and that which leaves
the myocardium is gone within a day or two
without any consequence.
So, we get very high levels of expression
of the adenovector within the heart resulting
in high levels of expression of FGF4. It appears
to bind to the receptor and stimulate the growth
of new blood vessels throughout the ischemic
region of the heart. That makes this approach
unique compared to the other approaches, which
have tried to deliver both the protein, which
has a very short half-life, or multiple injections
of the plasmid DNA, which are not taken up
by a receptor system. So, we think this system
is actually the best in terms of the approach
for growing new blood vessels in the myocardium,
and also very safe.
Finally, the degree
of change we see in terms of increased myocardial
perfusion on SPECT
is similar in magnitude to that we see when
we do an angioplasty procedure or a bypass
procedure. In some respects, we are essentially
performing a “biological bypass” in
these patients.
Q: This year marks the 30th anniversary of
coronary angioplasty and its inventor, Andreas
Gruentzig, said of his catheter-based technique
that what he really did was to demonstrate
for the first time that one could work safely
inside the artery of an awake, alert human
being. Would you say that the technique that
you're developing is an outgrowth of that?
Could this therapy be delivered in any other
fashion?
Dr. Moreadith: It really could not -- without
the advent of safe percutaneous interventions
within patients with coronary disease. Number
one, we wouldn't be able to save the lives
of patients coming in with an acute myocardial
infarction, and so the restoration of blood
flow to the myocardium has been the one thing
that's been proven to be of great benefit to
these patients. But, as you know, they have
progression of disease, and reach a point where
you can no longer do these percutaneous interventions
or bypass surgery. And that population of patients
is large – perhaps several hundred thousand – and
growing. Eventually, these patients all succumb
to refractory angina. This approach, using
a catheter down the coronary to deliver an
angiogenic gene, could not be done without
the precedent-setting work of many people including
Dr. Gruentzig, and Dr. Schatz, who was involved
in the development of these stents early on,
and many others who have spent much of their
careers advancing approaches of treatments
of patients with acute MI and refractory angina.
What we're finding is that the body has a
lot of innate mechanisms that, if we can find
a way to stimulate those endogenous and innate
mechanisms, we may be able to safely grow new
blood vessels within specific parts of the
body. This approach, angiogenic gene therapy,
and other approaches, including the recent
advances in cell therapy, are all catheter-based
systems that allow us to put these products
safely and effectively in the myocardium.
Q: What should people who think they might
be candidates for this study do?
Dr. Moreadith: If someone reads this site and
feels they may be a candidate for this study,
they can go directly to the Clinicaltrials.gov
site <LINK TO AWARE PAGE>, and they can
call me directly as the Chief Medical Officer
of Cardium Therapeutics, as the study's principal
investigator, and I can refer them, potentially,
to a site nearby where they could be evaluated
for entry in the study.
We also encourage not only patients who potentially
might be candidates for the study, but also
investigators who would be interested in participating
in the study, who feel they have a large population
of patients with refractory angina, particularly
female patients, they are encouraged to contact
us through the clinicaltrials.gov website as
well.
Q: So, at this point, but you're welcoming
other centers to get involved as investigators?
Dr. Moreadith: Yes, we are continually evaluating
centers that are both very experienced, and
have a large population of refractory angina
patients.
Q: This is an
exciting frontier and we’ll
all be watching how this turns out. Hopefully
patients or physicians reading this, who may
want to get involved, will contact you.
Dr. Moreadith: Well, I think you've done a
great job with your website, educating people
on multiple issues with cardiovascular health,
so I want to applaud you for doing that. It's
a great effort, and we definitely need more
of that, because people need to become aware
that there are experimental approaches out
there in late stage development - and maybe
one day there will be a therapy for these patients
as well. So, the more patients are informed,
the better.
|