Marc D. Feldman,
MD, FACC is Associate Professor of Medicine,
Director of Interventional Research and
the Cardiac Catheterization Laboratories at
University of Texas Health Science Center, San Antonio.
Besides his clinical practice, Dr. Feldman works in a number
of research areas, including nanotechnology, drug-eluting
stent hypersensitivity and optical coherence tomography (OCT).
In 2005, along with Thomas
Milner,
Ph.D.,
professor of biomedical engineering at UT Austin, Dr. Feldman
co-founded CardioSpectra to develop OCT technology for
clinical applications in the heart. The company
was acquired by Volcano Corporation in December 2007.
The coronary OCT device is not yet approved
for use in the U.S., but has just been used for the first
time
in
The
Netherlands.
The OCT images illustrating this interview
were
provided
from the "First
in Man Volcano OCT" performed at ThoraxCenter in
Rotterdam by Prof. Patrick Serruys and Dr. Evelyn Regar.
|
|
 Marc
D. Feldman, MD, FACC
Marc
D. Feldman, MD, FACC |
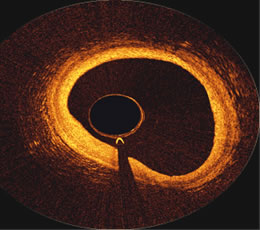
OCT
image inside the proximal portion of a Right Coronary Artery,
courtesy of
the First in Man Volcano OCT,
performed at ThoraxCenter
in
Rotterdam
by Prof. Patrick Serruys
and Dr. Evelyn Regar |
|
Q: What is OCT Optical
Coherence Tomography?
Dr. Feldman: It's like ultrasound --
only ultrasound is using sound, and OCT is using light. And
because light is so much faster than sound, it has a much better
resolution. So you can see details in the blood vessel with
light or OCT that you cannot see with sound. That's the main
reason it's considered an advance.
Q: Tell me a little
about how you developed the device.
Dr. Feldman: Actually OCT was invented at MIT in the early
'90s. It's been used in many different areas, but most of it
has been basic research. Our group at
the University of Texas was one of the first to develop a cardiovascular catheter
to use in the heart. I worked with Thomas Milner, who's
a professor of engineering, and a lot of the graduate students.
It actually took us ten years and we developed about
seven patents: on devices, catheters, techniques, and ways
to clear the blood.
In 2005 the University of Texas helped us spin out a company,
CardioSpectra, based on those patents. We found
local investors and the State of Texas gave us money as well.
Then, after three years, Volcano acquired our technology. |
Q: So how does this work? Is it an over-the-wire catheter?
Dr. Feldman: Yes. It’s just like intravascular ultrasound
(IVUS). It's over-the-wire. It spins just like intravascular ultrasound.
What makes it more difficult though is that, where sound can see
and reflect through blood, light cannot. Light is scattered by
the red cells, and so we have to clear the blood.
One approach,
taken by the Harvard/MIT group, is a device where you have to
blow up a balloon and occlude the vessel, and then flush it out
with
saline. People don't like that because the balloon itself may
cause some injury. So we're focusing on a faster imaging technique
or
physics, so that we don't have to occlude the vessel. We can
just flush with saline or contrast or both together.
Q:
How do you see this device being utilized during procedures?
Youve said
that OCT can actually see whether or not a stent has been
adequately
covered by endothelial cells.
Dr. Feldman: The device is not
FDA-approved still, so it cannot be used yet. But in the future,
it could be used to
look at patients who've got drug-eluting stents, where patients
are at risk for the stents clotting off, and causing heart
attacks. Although its not common, people feel that those patients
who don't have endothelialization of their drug-eluting stents
are those at risk for acute stent thrombosis. IVUS can't see
that, but OCT can. So one application will be in trying to
identify those patients who are at risk for acute stent thrombosis.
The other application, which is more futuristic, is trying
to predict heart attacks. We know at autopsy the patient characteristic
that the pathologist finds: a very thin fibrous
cap with a very big lipid core beneath. We know those
features can result in rupture of the fibrous cap which ends
in heart attacks. We can actually see the thin fibrous cap
with OCT. So were hoping we can predict future heart attacks
by looking at the plaques when patients have heart catheterizations. |
|
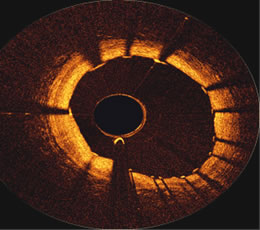
OCT image
just proximal to a stent in a Right
Coronary Artery, courtesy
of the First in Man Volcano OCT performed at ThoraxCenter
in
Rotterdam by
Prof. Patrick Serruys
and Dr. Evelyn Regar |
Q: What you’re describing is what’s often called “vulnerable
plaque”?
Dr. Feldman: Yes. There's a lot of discussion about vulnerable
plaque, but this would be the first tool to try to predict or locate
vulnerable plaques.
Q: My understanding is that OCT won’t actually be competing
with IVUS, because they complement each other. Don’t they
measure different types of things?
Dr. Feldman: Although light has better resolution, it cannot see
more than 2mm -- as opposed to sound frequencies, which can see
much deeper into vessel wall. So yes, in fact, the two techniques
complement each other.
What's fortunate for light is that a lot
of the vulnerable plaques are very superficial, within those
2mm, as well as the stents that
you’re looking at that are at risk for acute thrombosis.
So OCT is very useful for seeing the superficial features that
will predict heart attacks, and looking for stent features that
may cause them to clot off. But its weakness is that it can't see
very deep into the vessel wall, like 5 or 6mm, whereas ultrasound
can. So they really complement each other.
Q: So to recap -- to be able to actually
see the stent and determine if it’s healed and been covered by endothelial cells, there’s
been no way to do that until now?
Dr. Feldman: Right. Again because sound does not have the resolution
to see that. On the other hand, there are features of vulnerable
plaque, like excessive vasa vasorum – an enhancement of blood
vessels or the blood vessel itself -- light cannot see that because
it’s too far away, but sound (IVUS) will be key to be able
to see those features.
Q: So being able to see if the stent struts are covered, how will
that affect treatment of the patient?
Dr. Feldman: For instance, how long do you continue Plavix for?
The FDA just recommended extending it to longer, 12 months. But
for many of us, that's not even long enough. We still see patients
2-3 years out who are having acute stent thrombosis. You never
saw that with bare metal stents. The instance is only 1 in 200,
right? It's not very common, but does that mean every patient with
a drug-eluting stent gets put on Plavix forever? Or can OCT one
day say “Aha, that patient has thick endothelial coverage
on that drug-eluting stent. Therefore the risk of acute stent thrombosis
is very low. Stop the Plavix.”
Or, OCT has shown in Europe, where it's
being used more often right now because their regulatory agency
is more liberal, they're
seeing these necrotic cores behind the drug-eluting stents. So
in the vessel walls they’re seeing these necrotic cores that
have been seen pathologically. And they’re starting to see
those similar features in humans, in patients with drug-eluting
stents. Again they're not very common, but OCT can see these types
of features that IVUS just cannot.
Q: So, for example, if you discovered
a necrotic core that’s
behind a stent, what would you do?
Dr. Feldman: Well, one, you'd know that patient was at higher risk.
Two, you would never stop the Plavix; the patient would be on Plavix
and aspirin life-long. That's all you can do right now. The problem
is, if you have that condition and you're at high risk, if you're
that 1 in 200 patients, we don't have a good answer right now.
But at least it will reassure the other 199 patients that they
can stop their Plavix.
Q: I’m sure also that this
will be used a lot in research.
Dr. Feldman: For instance, the Endeavor stent was just FDA approved,
and there are going to be future drug-eluting stents that are
going to be FDA approved, and there's going to be a great desire
to study how much they endothelialize. And OCT could do that
in humans whereas IVUS just could not. So we've never had a tool
before that allows us to quantify the amount of endothelialization.
Q: Are these uses of the device something
that’s down the
line a bit, or might they occur quite rapidly, once the device
is approved?
Dr. Feldman: I think that once it's approved you'll see it appear
very quickly, but the market penetration may not be that different
than IVUS. You know the number of cases that it’s used on.
The bigger question is: will it predict future heart attacks from
vulnerable plaque? That's the bigger question. Now, if that's the
case, if it's successful in doing it, then the use of it will increase
tremendously because there's no way to predict a future heart attack
right now. And so that would expand tremendously its usage once
it appears, because anyone having a heart catheterization, most
patients have plaques that are really deep in the vessel wall.
You can't see them with angiography; you can't see them with IVUS
very well. And if you've actually seen one of those vulnerable
plaques with OCT, then that would expand its usage.
Q: So are you saying that you can’t
really see those vulnerable plaques with IVUS?
Dr. Feldman: No, not really -- the best you can do is that Volcano
has a way to look at plaque composition. But they still can't see
the thin fibrous cap. So in humans we know that at autopsy those
fibrous caps that rupture are usually about 30 microns thick. Sound
has a resolution of 100 microns, it can't even see down to that
resolution. Light has a resolution of about 10 microns, so OCT
can easily see that thin fibrous cap that's been ruptured.
Q: Is there any radiation involved with this?
Dr. Feldman: No, it's optical so it's just light, infrared light.
There's no radiation or X-rays.
Q: I’m sure that part of the
development will be getting the catheter small enough to get
into small narrow
vessels.
Dr. Feldman: Our system right now is about 1.1 mm -- a very low
profile; it's pretty similar to the current IVUS systems, so we
have it down to that level.
Q: Are there trials ongoing or about to start that will be testing
this?
Dr. Feldman: No. The goal is to just sort of get into the first
patients to get FDA approval right now. So there are no trials
that are ongoing at this time. So all these issues that we're talking
about right now, predicting heart attacks, for example, they're
very futuristic.
Q: Getting FDA approval is basically proving safety?
Dr. Feldman: The goal is to show safety, yes. IVUS is already FDA
approved, right? So one approach would be to sort of piggyback
it on to IVUS approval and apply for it as a sort of similar
device. That would probably make the most sense, because if IVUS
is a similar device and light is not toxic, then one would get
FDA approval by comparing it to IVUS.
Q: Do you see a time where you’d
have perhaps a dual catheter, using both imaging technologies
to get full information?
Dr. Feldman: That would make a lot of sense to combine the two.
Q: So, to sum up…
Dr. Feldman: It's a new tool. It's the first uses of infrared light
in medicine. It's actually in practice now in ophthalmology.
So if you go to ophthalmologists' practices, they're actually
using OCT to see not just to see the surface of the retina, but
into the retina. And my guess is you'll see it in the next ten
years, it will appear in colonoscopy and endoscopy, bronchoscopy.
So it'll be piggybacked onto other devices. But those will be
easier though. We did one of the harder problems first, because
we had the problem of the light scattering from the red cells.
If you’re in the eye, or the lung, or the G.I. track, it’s
just air or just clear fluid. So the problems we faced with scattering,
which are very difficult, just don’t exist in these other
organs. So that’s why it will just be a matter of time
before it appears in these other organ systems.
This interview was conducted in February 2008
by Burt Cohen of Angioplasty.Org.
|





