|
Q: In what percentage of cases do you use intravascular ultrasound (IVUS)? Dr. Foster: I personally use IVUS on at least 90% of my cases. My IVUS use is very high, perhaps one of the highest in the country. I’d preface that by saying I’m still about 90% drug-eluting stents, and my interventional population tends to be a more complex population than average. That just sort of reflects the way our practice runs. I’ll often do cases that are passed on by some of the other interventionalists in the practice and other cardiologists around the hospital or at other hospitals – they will refer cases to me that maybe they’re less comfortable in doing. So I’m intervening in a higher, more complex population than may be average. In general I recommend IVUS for complex lesions: bifurcation lesions, long lesions, diabetics, left main and particularly restenosis lesions. But I think there are very few cases where IVUS is not useful. I never see it as a liability to get the best information I can and I think IVUS gives me that. The lesions I would avoid doing IVUS in are situations where we have calcified tortuous anatomy, maybe hemodynamically unstable patients where it’s an emergency and you just need to get in there and get it fixed. Maybe a situation where we’re dealing with a small, very small artery, and there isn’t much doubt that the only size stent I’m going to be able to use is a 2.5mm drug-eluting stent and going in there and IVUS-ing a baseline is probably not going to change my mind. Q: Can you give me an example of how the use of IVUS changed your treatment
of a patient? So we did not place a stent. We showed her the IVUS and the angiogram and explained how she had no disease in her coronary arteries at all and she was discharged. It seemed her chest pain was an anxiety reaction and she was treated for that instead. If IVUS had not been used, she would have been implanted with a drug-eluting stent and would have had to be on aspirin for life and clopidogrel for at least 6 months to a year. So IVUS changed the intended treatment for this woman and she avoided all the complications and expense that would have occurred.
There’s no bringing in a machine, turning it on, waiting for the computer to boot up, and then a technician adding the name, the age, birth date, none of that has to be done. Our system is a Volcano system integrated into a GE Innova 2100 lab, so the IVUS is always on -- if the cath lab’s on, the IVUS is on. So when I start my procedure, and I get to the place where I’m ready to IVUS, it’s literally handing the end of the ultrasound catheter off the side of the table to a technician who plugs it in, and you’re ready to go. I would call it a plug-and-play environment, and you instantaneously have your pictures. In about one minute, I can get the IVUS set and do a pull-back into the artery that I’m interested in. And, of course, I’m looking at the images as I’m pulling back, and I’ll usually spend another minute or two, on an interesting case, manipulating images, maybe making some measurements, and so forth. So from soup to nuts, we’re talking about three minutes to do IVUS.
Q: Tell me technically about the integrated cath
lab – can
an existing lab be retrofitted or does this have to be built-in from the
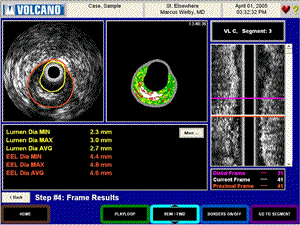
start? Q: How does the integration of IVUS and fluoroscopic angiographic images help? It really helps me figure out where to land my stent. We’ve known for a long time that you want to land the distal end of your stent in as normal an artery segment as you can. Of course, the problem with angiography is we underestimate mild disease. So sometimes the vessel looks pretty good just beyond the lesion, but when I’m looking at that same region on ultrasound, many times I can see pretty severe disease in there. So I actually mark the area of artery in my mind that’s really pristine by IVUS. And then I know I’ve landed that stent into the best possible segment I can. In the future there will probably be this type of integrated IVUS-angiographic system called AIM, which stands for Angiographic IVUS Mapping, I believe, so you could look at the angiogram and see where your lesion is, put a cursor on there and the IVUS image would automatically link right to that spot. That’s sort of what I’m doing in my mind, when I do these pullbacks in the integrated lab. Once I finish that 1 min, 90 sec, baseline run, I know exactly where I want to put my stent. I know exactly the size of the vessel I’m dealing with. I know my vessel’s 4.0mm, and I’m putting in a 3.5mm drug-eluting stent which is the largest we have available, so I know I’m going to have to go in after I deploy that stent with a 4.0mm balloon and I can do that very rapidly before I even bother to take any more angiograms to assess my results. So I get a tremendous amount of information for a 1-2 minute assessment that guides the rest of my case. And, of course, when I finish my stent, I always re-ultrasound to be sure that the stent is deployed like we want. I just think the integration speeds up the IVUS time so quickly that I think it will lend itself to more expanded uses of the system. Because time, I think, is really a prime obstacle for many people using ultrasound.
Three, I use the Virtual Histology feature to quickly measure the vessel diameter and lumen diameter because the software automatically performs edge-detection and gives me this info at the touch of the VH button. Finally I sometimes use VH to plan out my stent strategy. If there is a calcified plaque, or fibro-fatty atheroma, I might pre-dilate or use a cutting balloon to remodel the vessel prior to stenting and give the stent a better situation to expand in. Also, I will get the diameter information, so I know ahead of time that I might, for example, have a 4.5mm vessel diameter, so I’m going to need an extra 4.5mm balloon to completely expand the stent. Q: Are there any other ways in which IVUS has impacted your treatment decisions?
So we started IVUS-ing and I saw that many of these stents were just not well-expanded in the artery. So we addressed that, went to more aggressive post-dilation, and after many months went by, we realized we had not seen any more stent thrombosis. That really started my curiosity as well as my habit of IVUS-ing these drug eluting stents. Because our experience is that these stents are harder to expand than the thin-strutted bare metal stents. Q: Why are drug-eluting stents harder to expand? Q: Dr. Marco Costa recently published a study
stating the figure for under-expanded Cypher stents at 66%. Is this really
accurate? It sort of reminds me of the early days of when we started putting in bare metal stents. We had this absolutely amazing anti-coagulation regimen. We’d use Dextran in the lab, heparin in the lab. Then, as soon as we put the stent in, we’d pull the sheath. Then we’d go on Ticlid, Coumadin and continue on heparin intravenously until the Coumadin was therapeutic. And for the first six months that we were putting these stents in, the average hospital stay was 7-8 days. It exceeded bypass surgery. There were tremendous bleeding problems, and so forth. And I thought, “These stents are just unacceptable.” Then Antonio Colombo discovered using IVUS that we were under-expanding those bare metal stents -- that if you really expanded them out well in the artery, you didn’t have to do the heparin, you didn’t have to be on Coumadin. You could just be on aspirin and Ticlid (subsequently we used Plavix) and you could get patients out in 24 hours. It totally changed the way we thought of stents when this was discovered. I think in a sense we’re going through this same situation with the drug-eluting stent. I don’t think we’ve had quite an appreciation of how to optimize its implantation and I think once we’ve discovered how, we’ll see the true potential of drug-eluting stents in the clinical realm. And with what’s happening now with the integrated system, it’s going to be harder to ignore IVUS or excuse not using it because the time factor to do an IVUS examination becomes essentially negligible. This interview was conducted in June 2007 by Burt Cohen of Angioplasty.Org. |