
Augusto ("Gus") Pichard,
MD, specializes in Invasive Cardiology and is board certified
in Internal Medicine, Cardiology and Interventional Cardiology.
He joined Washington Hospital Center in 1983 as director
of the Cardiac Catheterization Lab. He is also a Professor
of Medicine at The George Washington University Medical Center.
Dr. Pichard has written more than 500 manuscripts for peer-reviewed
journals on many topics in Invasive Cardiology, including
innovative heart disease treatment techniques. He is also
very active in national and international educational activities
related to his specialty.
Prior to coming to the Hospital Center, Dr. Prichard directed
the Cardiac Catheterization Lab at Mount Sinai Medical Center
in New York, N.Y. He also completed a Cardiology fellowship
at Cleveland Clinic and served on its staff until 1975.
|
|
 Augusto
D. Pichard, MD
Augusto
D. Pichard, MD |
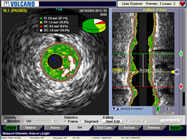
Intravascular
Ultrasound Image |
|
Q. For what percentage of
your cases do you use either IVUS or FFR?
Dr. Pichard: I use
IVUS on all my angioplasties, always! The IVUS is already set
before I come into the cath lab and I always image, both before
and after. It keeps me humble. The IVUS teaches me so much;
I change the strategy all the time based on IVUS. It makes
angioplasty, easy, uncomplicated, and very successful.
I always
say I save the hospital money because, by knowing exactly
what I'm doing, I have less complications. I rarely need
to add
another stent. So that way, the hospital makes money: less
complications and less need for a second stent, which is
not reimbursed. |
We use a lot of FFR. If the lesion is a difficult interpretation
on the angio, and the IVUS gives me a borderline measurement, I
do FFR. We used to stent everything with less than 4 mm square
on IVUS, now I've learned that you can have 3.7, 3.8 and still
not have a physiologically significant lesion. So now we've modified
our strategy and do not stent those.
Q. The FAME study obviously had some impact there?
Dr. Pichard: Right. An abstract was presented at this past AHA:
IVUS vs. FFR to better understand the correlation, and some of
these new discrepancies. I am a great believer in the physiology;
I think the FFR has the upper hand, especially now that we can
manage, medically, the intermediate lesions. Five years ago,
eight years ago, you had a 50% lesion that looked ugly, it was
safer to stent it. But I think that's changed now, as a result
of the enhanced medical therapy.
Q. This past
year, the ACC/AHA/SCAI issued some new guidelines giving
FFR
a higher
level of evidence
in PCI and STEMI cases. Its now a Class 2A indication. Theyre
recommending that FFR be used more, not in every lesion, but
where there
are questions, or intermediate lesions. Do
you think this will have an affect on practice in the U.S.?
Dr.
Pichard: It should give the physicians more confidence that
they are using something that is approved that is in the
guidelines. My concern still for some is the lack of reimbursement.
If someone uses it a lot, the cath lab director is going
to be after them for using so much of it. I don't seem to
have
that problem, because of proving to the hospital that we
saved them money by doing optimal treatment to begin with. |
|
Fractional
Flow Reserve
(FFR) Wire |
Q. So do you think the reimbursement for IVUS and FFR and these
types of measurements needs to change?
Dr. Pichard: Yes, definitely.
Q. In Japan IVUS is reimbursed by itself, as a stand-alone part
of the procedure, correct?
Dr. Pichard: Right.
Q. In all of the DES clinical trials that were reported from Endeavor,
Xience, all of them, the Japanese arms always seem to have better
results. No matter which stent they were using, they seemed to
have less restenosis. I asked Dr. Shigeru Saito, the principal
investigator on many of these studies, about that and he said,
it's because they use IVUS. Do you think that's a fair comment?
Dr. Pichard: That's a very fair statement. It's amazing how much
I see among those that don't use IVUS. We have an open lab, so
a lot of doctors call me to assess something. And I will use IVUS
and I realize what an inadequate job has been done, although the
angiography was acceptable. I’m all for understanding; there's
nothing like knowing what you're doing. It's fascinating that,
with all the trials that we've done, IVUS has not come out as a
black-and-white winner. You would expect from what I say that all
the trials would show much better outcome with IVUS. But it's been
difficult to prove it. Part of it, I think, is because it's been
so expensive. So physicians use it sparingly. So when they use
it, they don't know how to use it well! There is an element of,
like anything in life, if you use very rarely, you don't know how
to use it best and you don't get all the benefits.
Q. So you need to use it regularly and you need to be trained
on it more?
Dr. Pichard: If you're well-trained and use it regularly, then
you get to understand what everything means. It's not just measuring
diameter or length. You get to understand better plaque distribution,
plaque thickness; you see that there is no calcium, but there is
shadowing. That’s a very hard fact: all these little intricacies
of a technology that is sophisticated.
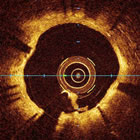
OCT (Optical
Computed
Tomography) Image |
|
Q.
What about OCT? Do you see that opening a new era? Or is
that still in the
future?
Dr. Pichard: OCT is fascinating, it gives us such good
resolution, we understand so much better what's going on in
that very thin segment of the vessel wall. We know exactly
what's going on in the lumen. The clinical relevance will have
to be proven. What this is doing is teaching us much better
to understand what we do. Once we understand what we're doing,
we may not need to image everyone, but it's a fascinating look
into the vessel. In Spain I did a vein graft recently with
the hypothesis that I always use a small stent in a large vein
to minimize plaque prolapse and embolization. And on the angio
it was a fantastic result, so it was on the IVUS. But on OCT
I found a lot of plaque prolapse, which the IVUS did not show.
So it makes you understand better each technique, each approach.
With OCT, I hope they will be able to come up with a fiber
that is not very expensive that people can use a lot and we'll
all learn from it. |
Q. What are you doing right now, things that might be new that
we haven't heard about in the area of intravascular imaging?
Dr. Pichard: I think we are going to redefine the significance
of a minimal lumen area by IVUS. We have that 4mm square break
point that we've used for over 10 years and I think we are going
to have to modify that and we are going to understand, thanks to
FFR, according to lesion length and vessel diameter, what is the
true ischemic minimum lumen area. So that 4mm square will no longer
be what it has been in the past.
Q. It might be on a lesion by lesion basis?
Dr. Pichard: In a small vessel it might be 3mm, so 3.5mm is not
significant; in a very small vessel, maybe 2.5mm square.
Q. What would you recommend to an interventional cardiologist
who doesn't use the tools of FFR and IVUS? Is it something he or
she really should do and how to learn?
Dr. Pichard: Definitely. I've seen two models - we have groups
that come and spend 3-5 days with us and they see a lot of IVUS
in one week at the hospital center. We give them talks on it and
they get exposed to a lot of decision-making based on IVUS. That's
one option. The other option is to take one of those two-day courses
dedicated to it. There is a dedicated course on FFR in Nice that
the European Society of Cardiology organizes -- and there are others.
So they become familiar with the subject in depth, and then when
they do it they feel confident, they know exactly how to do it
and how to interpret it. Once they have these tools they can use
them in their angioplasty practice to great benefit for the patients
and themselves.
Doing IVUS removes all the stress from
angioplasty, in my opinion. I do all direct stenting. I first
do IVUS and once I have my measurement
and the plaque characteristics, I know exactly what stent to put.
If the plaque is very hard and is going to need Rotablator or a
cutting balloon I do that first and then I do the stent. If the
vessel is 3.5mm, I’ll downsize the stent to 3mm or 2.75mm
and I bring it to high pressure, 18 or 20, and I now have plenty
of room go out there, so I haven't had a perforation in many, many
years, but I get beautiful expansion -- so all of this makes angioplasty
free of stress and, in my opinion, fewer complications, safer.
Q. Could you talk a little bit about what spot stenting is, and
specifically the ways that it involves having to be able image
things in a different way?
Dr. Pichard: It's a very exciting hypothesis, and it’s based
on the new knowledge regarding coronary disease. The new knowledge
is that, based on COURAGE and FAME plus a number of IVUS studies,
we can achieve plaque stabilization, even plaque regression, with
optimal medical therapy, including high dose statins, Plavix, etc.
The older work of the RAVEL era is no longer needed; it’s
over. When RAVEL came out we were told you need top put a drug-eluting
stent from normal to normal, because if you leave plaque on the
edge there will be a lot of restenosis or thrombosis.
And I reviewed that very carefully; the data published on edge
restenosis goes from zero to 5% at the worst, most of them are
about 2% incidence of edge restenosis. And the edge restenosis
is determined by the amount of plaque that you leave. If you leave
more than 50% plaque burden at the edge, then there is more likelihood
of restenosis. It's not much, but those are the ones that get it.
So based on the two concepts then, I thought maybe there is no
need to cover the entire plaque; we can just cover the tight segments
of a long lesion.
In addition to the new understanding of
the evolution of intermediate plaque, is the fact that we know
now that the longer the stent,
the more metal we put in, the more polymer we put in, the more
drugs -- the more problems for that artery, all kinds of problems:
more thrombosis, more restenosis. The physiology of the vessel,
we understand, is altered and, of course, if some day that patient
needs other interventions, like bypass surgery or more percutaneous
interventions, that vessel is already sacrificed, it's already “metalized.”
Two or three years ago I started using the concept of spot stenting:
attend to really tight lesions and leave the rest alone. And I
do this guided by intravascular ultrasound, to make sure that what
I leave alone is truly an intermediate lesion and not something
severe that I just don't see on angiography. And the results have
been outstanding. There is no increase in restenosis or thrombosis.
Q. There was a randomized study published about the spot stenting
technique?
Dr. Pichard: It was over 150 patients and was published in the
American
Journal of Cardiology. That's the only randomized study.
In addition to that, I have experience at the hospital where we've
been doing this for three years, with mostly my cases, and the
outcome is very good. We’re looking at the database and there
is no hint of increased events doing it this way. For many, many
patients, most of my patients get the least amount of metal that
is necessary and we see no sign of increased events. So I'm excited
about that. I think it's a good strategy that goes along with modern
thinking that optimal medical therapy is very effective. Of course
to do it optimally, ideally you need either FFR or IVUS or maybe
even OCT. I have not done this with OCT. But knowing what OCT shows,
it would also be very helpful to this type of work.
What I look at with IVUS is to make sure
that at the edge there is no more than 50% plaque. If there is
dissection I never put
an additional stent, unless there is an occlusive dissection. What
I routinely do for edge dissection, I put the same balloon from
the stent, a low pressure, 4 or 5 atmospheres and leave it there
for two minutes. Invariably it fills in nicely and again, it's
not associated with later events. There are a few publications,
some of them from our group showing that edge dissection is not
associated with events unless it is an occlusive dissection. With
IVUS this becomes a very simple and safe way to do it, because
you know exactly what's going on. You know if the plaque is left
at the edge, if it’s an area of remodeling, you know if you’ve
got rupture, you know if there's thrombus, and you can act accordingly.
Q. IVUS or possibly FFR or OCT are very important. You couldn't
really do this without some kind of new imaging technique?
Dr. Pichard: You could, but it's much superior to do it with an
imaging technique. The new knowledge has shown that angiography
is not an accurate way to access lesions. There is a recent paper
in Circulation with intermediate left main stenosis and FFR showing
the same constant, that 20-30% of what looks severe on angio, was
not judged significant. So there are both extremes: some severe
angiographic lesions on the left main were not severe by FFR and
some minimal lesions on angiography are actually severe on FFR.
So from FFR and IVUS we have learned that angiography misdiagnosed
about 1/4 to 1/3 of lesions, re-enforcing the concept that good
angioplasty should be guided by some form of imaging. You can always
get a beautiful print to give the family. But that's not what I'm
after. I'm after 5 and 10-year excellent outcomes. And that's why
I'm trying to put less metal, less polymer in the artery so that
the physiology is not so affected, so there are less complications
in the long term. So the vessel is left open for future interventions
if at all needed.
This interview was conducted in November
2009 by Burt Cohen of Angioplasty.Org.
|





