 |

|
 |

As coronary imaging turns
to intravascular technologies -- views from inside the
arteries themselves -- new fields of research and clinical
study have
opened. One of these new technologies is Virtual Histology
IVUS and Angioplasty.Org recently sat down with one of the
leading researchers in VH IVUS, Dr. Szilard Voros.
Dr. Voros is the medical director for Cardiovascular
Magnetic Resonance (CMR) and Computed Tomography (CCT)
at the Fuqua
Heart Center of Atlanta at Piedmont Hospital,
dedicated to providing world-class non-invasive
imaging of the heart and blood vessels. Dr. Voros utilizes
the latest technology to detect cardiovascular disease
and collect life-saving information available in the past
only
through invasive procedures, such as cardiac catheterization
and invasive coronary artery angiography.
With years of experience in these new and exciting fields,
Dr. Voros has published extensively in clinical as well as
investigational aspects
of CMR and CCT. He is a founding member of the Society
for Cardiovascular Computed Tomography (SCCT) and
is a member of the Society for Cardiovascular Magnetic
Resonance
(SCMR). Dr. Voros has published extensively in the field
of advanced cardiovascular imaging and regularly lectures
at national and international meetings. |
|
 Szilard
Voros, MD
Szilard
Voros, MD
Fuqua Heart Center at
Piedmont
Hospital, Atlanta, Georgia |
Q: Cardiologists certainly are familiar with intravascular
ultrasound (IVUS) but what is Virtual Histology IVUS, or VH IVUS,
and how does it work?
Dr. Voros: It's an ultrasound-based technique. Ultrasound is obviously
used for a lot of different applications. But this just happens to
be intravascular, so it's a small ultrasound probe that actually
is placed on the tip of a catheter that can be then advanced into
the arteries that feed the heart. Once the transducer is inside the
artery, it functions just like any other ultrasound, except obviously
since it's inside, it's taking pictures from the inside out as opposed
to from the outside.
It's an ultrasound probe so it sends out
ultrasound frequencies, sound waves, and then these sound waves
are reflected
back from
different components of the vessel wall, which is what we're interested
in looking at. When these sound waves bounce back, much like an
echo as when we're screaming in the mountains, then we can detect
these waveforms that are reflected back. And there are several
features of the sound waves that come back that we can measure.
For example, we can measure the amplitude and the frequency of
these sound waves. Specifically using the amplitude is how we create
the grey scale images in standard IVUS.
Now what VH does is look at a different layer of information in
this reflected sound wave information that comes back. This so-called
radio frequency backscatter information is then translated into
a color scale with the idea behind it that different tissues have
different characteristics as to how they reflect the sound waves
back. So, as we unravel this radio frequency backscatter information,
it gives us an idea of the composition of the different tissue
types within the vessel wall. That's the general idea.
Q: Is there special equipment
or hardware needed to produce VH IVUS, or is it mainly a
software interpretation
of the same
data
you’d get from a standard IVUS imaging catheter?
Dr. Voros: There are several vendors out there that make IVUS catheters.
Essentially the way you interpret the information that comes back,
some of that is hardware, but most of that is software interpretation.
Specifically VH-IVUS is an algorithm only available from one of
the vendors.
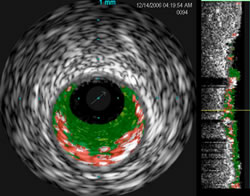
VH IVUS
shows color-coded plaque composition:
red is necrotic core,
dark green is fibrous, light
green is fibrofatty and white
is calcified plaque.
The grey circle around these plaques
is the
vessel wall. The black inner oval is the open
diameter
of this blocked artery |
|
Q: Four types
of plaques are listed as to what VH IVUS can identify:
dense
calcium, fibrous,
fibrofatty and necrotic. What are those and what are the clinical
implications for the patient.
Dr. Voros: Actually those particular
classifications are vendor-specific, however, those are general
components of plaques that, if you look at a histological section
of an atherosclerotic plaque, which we do all the time in our
research efforts, we find the same components. Obviously the
nomenclature is corresponding to that. In fact VH-IVUS was
validated and the algorithms were developed on the basis of
these histological sections.
Essentially the quick overview is
that atherosclerotic plaques develop because of two primary
processes: number one is what we call lipoprotein deposition
in the plaque.
It's just the cholesterol-rich particles that are deposited.
That's the primary process. But this process also fuels the
recruitment of inflammatory cells, so inflammation is the
second component.
These two processes go hand-in-hand for a while. |
| But then, as those processes
progress, part of this plaque on the inside of the vessel wall
starts to undergo what's call apoptosis or necrosis, meaning
the cells start to die. So you have this necrotic core within
the plaque. So that's essentially the area where there are
no cells left -- only the cholesterol debris and the cholesterol
crystals and a lot of the cell debris. As that process continues,
the cap that separates the plaque from the blood pool gets
thinner and thinner, so you can have micro-ruptures of that,
you can have small areas of bleeding or hemorrhage into the
plaque. Eventually, the healing of the small micro-hemorrhages
and some other processes may result in calcification. So in
general the way to think about this is that calcification is
a later stage manifestation of atherosclerosis. A lot of people
think of calcification as actually the healing process per
se. So, what we know about are the non-calcified components,
which are the necrotic core, the fibrous and the fibro-fatty
tissue, those are the non-calcified areas. Typically they are
associated with earlier stage atherosclerosis and in general
more vulnerable plaques. Whereas calcification typically represents
later stage disease and actually a healing process. |
|
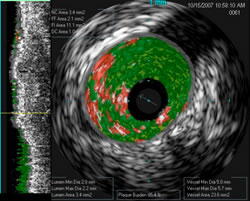
Another
VH IVUS image shows a vessel with
85% plaque burden -- the
actual vessel, shown
by the grey oval is around 5mm, but
the
opening, shown as the black inner oval, is
only 2.1mm.
Virtual Histology reveals the
composition of the significant
plaque, which
cannot be seen on a standard angiogram. |
Q: There's a lot of controversy about what causes thrombus or
clot to form in the coronary artery, resulting in an acute myocardial
infarction (AMI) a.k.a. a heart attack. On an angiogram, you see
a blockage. But recent discussions have been focused on this thin
capped fibrous atheroma (TCFA) or so-called vulnerable plaque,
which could rupture, spill lipids into the blood flow and quickly
cause thrombus to start forming.
Dr. Voros: The way I look at that is there's this paradox: the
current paradigm in cardiology, cardiovascular disease, is what
I refer to as the hemodynamic paradigm. And what that paradigm
says is that if you have an obstructive lesion greater than 70%
diameter stenosis, then that leads to flow limitation. Therefore
you have ischemia which causes you chest pain. If I relieve this
obstruction by angioplasty, then this ischemia goes away, the perfusion
is then normalized, so your chest pain goes away, and you live
longer. This is the paradigm that framed our thinking in cardiology
for 30 years which is the basis of angioplasty and bypass surgery.
The paradox is what we learned back in the 1980's from the thrombolytic
literature; back in those days we did not do primary angioplasty,
we did thrombolytics -- when we gave thrombolytics, the clots resolved.
So when people looked at arteries that were closed at the time
of the acute infarction, oftentimes the underlying culprit lesion,
on which the thrombus formed and which occluded the artery, the
underlying culprit lesion in the majority of cases was a less than
70% stenosis. In other words there was this paradox where we knew
what to do with the obstructive plaques. However, up to 70% of
these infarct-related lesions were actually non-hemodynamically
significant.
So to that effect, we're conducting the
ATLANTA study in my lab, which is focusing on non-obstructive
plaques. We’re studying
these non-obstructive plaques between 40-70% very carefully. We're
characterizing these plaques in the CT labs, using CT coronary
angiography. Then we take the patient to the cath lab. In the cath
lab we measure the hemodynamic significance of the lesion by performing
Fractional Flow Reserve (FFR) to truly understand the hemodynamic
significance. Then we do IVUS and VH-IVUS to look at the composition
of the plaque.
It's a longitudinal study so we're following people up and so
we're trying to understand what is the significance of having,
for example, VH TCFA or VH necrotic core and trying to understand
what is the natural history of disease. From retrospective studies
we know that when people compared so-called stable plaques vs.
unstable plaques, meaning plaques that had events associated with
them or not, when they retrospectively compared their baseline
features, in general, lumen area, lumen diameter, percent diameter
of stenosis, percent area of stenosis did not differ from unstable
to more stable plaques. But things like positive remodeling, plaque
burden, such as percent atheroma volume, were higher in the unstable
plaques. Also from a VH composition perspective, these so-called
unstable plaques had more TCFA and lipid-rich necrotic core, compared
to the stable lesions.
Q: And the different between stable and unstable lesions can be
visualized how?
Dr. Voros: These were retrospective studies, so researchers looked
at patients who a year or two before had an IVUS and VH, and in
the following one or two years they had an event related to a plaque
or not. And then they went back retrospectively to look at the
underlying features there. So it was clinical definition for stable
vs. unstable plaques. Now these retrospective studies told us,
okay if you look lesions that ended up causing an event, in general
they were more positively remodeled, in general had more percent
atheroma volume, in general had more necrotic core and in general
had less calcium than lesions that did not cause events. The difficulty
at this point is that we see a lot of those lesions that are positively
remodeled, have a lot of PAV (percent atheroma volume), have a
lot of necrotic core, and some of them do go on having events and
some of them don't. So we still need to further refine some of
these features to predict events.
Q: Where is the ATLANTA study at right now, and what does the
acronym stand for?
Dr. Voros: We are in the second phase of the study. The first phase
focused in patients with 40-70% lesions. The second phase is focusing
on the high grade lesions over 70%. And the acronym stands for:
Assessment of Tissue
Characteristics, Lesion Morphology and
Hemodynamics by Angiography with Fractional
Flow Reserve, Intravascular
Ultrasound and Virtual Histology and Non-Invasive
Computed Tomography in Atherosclerotic
Plaques.
Q: I see why this study needs
an acronym. You’re using virtually
every imaging technology that exists. A few years ago, Prof. Patrick
W. Serruys of the Thoraxcentre in Rotterdam said, "Plaque
imaging using VH IVUS will provide key information and may shift
the paradigm of how we diagnose and manage patients with cardiovascular
disease." How close are to Prof. Serruys’ prediction?
Dr. Voros: We're getting there. I think we're learning and, as
with most everything, it's not as simple as we originally thought.
But nevertheless, here's my underlying take on VH IVUS. There has
been some discussion as to how accurate it is. When you actually
look at VH IVUS and then you look at human tissue, how accurate
is the algorithm in classifying parameters. In some of the original
work it was pretty robust. There were sub-studies published recently
that said maybe it's not so robust in characterizing tissue. From
a clinical perspective it doesn’t really matter, as long
as whatever you see does in fact predict clinical events. I think
that we don’t have that answer today yet and I think we have
to have the PROSPECT study completed before we can tell whether
or not Patrick's statement is going to be a reality or not.
Q: Are you involved in the PROSPECT
study?
Dr. Voros: We were part of the PROSPECT study site here at Piedmont.
They also collect a subgroup of PROSPECT who had CT angiography
as well as VH IVUS. Our ATLANTA study is sponsored by the same
organization that funds PROSPECT, so there has been some discussion
in potentially combining the data sets.
Q: Is VH IVUS still basically a research tool?
Dr. Voros: Yes. I think that's a fair statement. Of course, it
is an FDA-approved product and I do know that some physicians
make their treatment decisions based on their VH IUS, but honestly
there has not been a single randomized prospective controlled
strategy trial that looked at the outcomes from whether or not
you made your decision based on VH IVUS or not.

Piedmont
Hospital
Atlanta, GA |
|
Q: Do you or the people
in your department find VH IVUS useful for making clinical
decisions?
Dr.
Voros: We most certainly look at it. We most certainly take
it as one piece of the puzzle. There are a lot of things
we know about the
patient: we know their clinical history, we know typically
the myocardial perfusion and we have the geometry of the
plaques, and we do take this into consideration. So clearly
there are
cases when we have altered the therapy on the basis of VH
IVUS.
I still feel that some strategies need to be performed,
that
is when you randomize people and you make your decision
on the basis of VH IVUS or not and look at outcomes. And
not
necessarily hard endpoints. It could be as simple as the
appropriate length
of stenting, that kind of stuff. |
Q: Or whether to stent or not to stent?
Dr. Voros: Whether to stent or not to stent, that is the ultimate
question! We just have to have the natural history trial first
before we do the treatment trial -- and the natural history trial
is PROSPECT.
Q: Because it's not clear whether stenting a vulnerable plaque
before it ruptures is clinically beneficial or not?
Dr. Voros: That's the big question and it is the question today
and that's exactly the question that PROSPECT and our ATLANTA study
are trying to get at. We have plans, based on our ATLANTA study,
that if we reach certain parameters, we would move forward with
just that study which is taking hemodynamically non-obstructive
lesions, and on the basis of some pre-specified characteristics,
based on CT characteristics and VH IVUS characteristics, we would
plan to randomize people to stenting vs. no stenting in the non-obstructive
lesions. But that's where the frontier is today!
Q: Because there's so much controversy generated, for example,
by the COURAGE trial -- does stenting and angioplasty improve outcomes
or not?
Dr. Voros: That's right. I think VH IVUS has a huge potential obviously
of the intravascular plaque characterization techniques now that
are available that are FDA-approved. There are two of them right
now, VH IVUS and the near-infrared spectroscopy. Then there’s
OCT which is not yet FDA-approved. Of those techniques, quite honestly
VH IVUS is the most mature and we collectively have a very extensive
experience with that. I think we can start to make some conclusions,
but again I think the PROSPECT is critical to have the follow-up
data from PROSPECT.
Q: When is PROSPECT data to be released?
Dr. Voros: I think that TCT next year is the plan.
Q: Dr. Voros, thank you for your time.
(More information about VH IVUS can be found
at www.vhivus.com --
a web site from Volcano Corporation.)
This interview was conducted in November
2008 by Burt Cohen of Angioplasty.Org.
|






