|
Boston Scientific's
Carotid Artery
WALLSTENT® Gets FDA Approval
Device Enters Market That
Has Been Challenged by Reimbursement Issues
|
 |
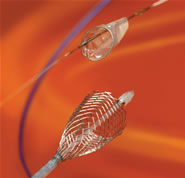
Boaston Scientific's
Carotid
WALLSTENT with FilterWire |
|
October 23, 2008 --
Today the U.S. Food and Drug Administration gave its approval
to Boston
Scientific's Carotid WALLSTENT® Monorail® Endoprosthesis for
the treatment of patients with carotid artery disease who are
at high risk for surgery. Less than 48 hours since the FDA lifted
most of the major restrictions of its 2006 warning letter to
the device manufacturer, one of their major products now has
been given the green light. In a press release, Boston Scientific
stated that its Carotid WALLSTENT Endoprosthesis is "the leading
carotid stent in Europe and other international markets", and
that the company plans to launch the product immediately in the
United States. |
It has been over three years since Dr. Christopher
White of the Ochsner Clinic in New Orleans presented the one-year
results of the BEACH Trial, a clinical trial that measured
the success of the WALLSTENT. In the interim, several other
carotid stent devices from Abbott, Cordis, eV3, and even an
earlier device from Boston Scientific, the NextStent, have
received approval.
However, the entire issue
of carotid stenting received some unwelcome news last week,
when the Centers for Medicare & Medicaid
Services (CMS) decided not to change its relatively restrictive
reimbursement guidelines, currently limited to symptomatic
patients at high risk for surgery with a carotid blockage of
70% or more. This patient population is actually smaller than
the patient group approved by the FDA -- so the current situation
is that there are patients for whom the FDA feels this non-surgical
treatment is safe and effective, but for whom Medicare will
not pay.
|
|

Christopher
White, MD
|
The decision by Medicare was criticized by a number
of groups, notably the Society
for Cardiovascular Angiography and Interventions (SCAI),
which stated:
"In making its decision, CMS cited a lack of
published data on the benefits of CAS. However, the decision
came just one day before the
latest data from the Stenting and Angioplasty with Protection
of Patients with High Risk for Endarterectomy (SAPPHIRE) Worldwide
Registry
was published in Catheterization and Cardiovascular Interventions.
SAPPHIRE demonstrated that CAS is as safe and effective as CEA
in high-risk patients and continues to show comparable results
after
30 days follow up.
"The SAPPHIRE results provide further
support for the benefits of CAS compared to surgery for patients
at increased risk of surgery
due to both comorbid conditions and anatomic high-risk features
whose carotid arteries are severely narrowed,” said Christopher
J. White, MD, FSCAI, Chairman, Department of Cardiology, Ochsner
Clinic
Foundation, New Orleans. “There is now compelling, published
evidence that CAS is a good option for patients at risk of stroke.
"Additional peer-reviewed studies expected
to be published this month may further support the benefits of
CAS. Recent data from the CAPTURE
and EXACT trials examine the safety and effectiveness of two
brands of carotid stent and embolic protection systems. If new
data continue
to support CAS, SCAI will work with CMS to expand coverage of
the procedure so more patients can benefit from a wider spectrum
of treatment
options.
"Stroke remains a leading cause of death
and disability in the United States, but new, less-invasive treatments,
including carotid
artery stents, are helping save lives,” said Bonnie H.
Weiner, MD, FSCAI, SCAI immediate past president. “Patients
deserve every possible option to help prevent stroke."
Indeed many were taken aback by the CMS pronouncement,
literally days before the much-anticipated results of the SAPPHIRE
study were published.
The SCAI has created a
web page with sample letters for patients
and physicians to send to their Congressional Representatives.
As for the WALLSTENT, Boston Scientific feels that their device
will become a major player in the carotid stent field. Their press
release states:
The Carotid WALLSTENT is a self-expanding stent mounted on a rapid
exchange delivery system, designed to re-open the carotid artery
by treating stenoses, and improve blood flow to the brain. The stent
features a closed-cell design, engineered for excellent lesion coverage
and angiographic results. The system is designed to be highly deliverable
and provide access to the toughest lesions.
It is used in conjunction
with the FilterWire EZ™ Embolic
Protection System, which is designed to capture plaque debris
released during the stenting procedure, preventing it from
traveling to the
brain, where it could create an increased risk for stroke.
The device features simplified filter sizing - accommodating
vessel
diameters
between 3.5 mm and 5.5 mm - and offers efficient preparation,
deployment and retrieval.
| "The closed-cell design
of the Carotid WALLSTENT Endoprosthesis is intended to provide
increased scaffolding for optimal lesion coverage and a smooth
inner lumen," said Barry T. Katzen, M.D., Medical Director,
Baptist Cardiac and Vascular Institute, Miami. "This feature
will make the Carotid WALLSTENT an attractive new treatment
option for U.S. physicians and their patients." The Carotid WALLSTENT Endoprosthesis with the FilterWire EZ
System is the only carotid artery stent system approved in
the United States with an indication that includes the treatment
of bilateral carotid artery disease (blockages in the carotid
arteries on both sides of the neck).
|
|

Barry
T. Katzen, MD |
The carotid arteries are the main conduits through which blood flows
from the heart to the brain. Carotid artery disease occurs when fatty
plaque builds up inside the vessels, causing them to harden and narrow,
which increases the risk of stroke. Most patients with carotid artery
disease are treated with carotid endarterectomy, a surgical procedure
involving an incision in the neck and removal of the plaque from
the vessel walls. Carotid artery stenting is a less-invasive alternative
in which a stent is delivered to the site of the blockage and expanded,
forcing open the walls of the arteries and restoring blood flow.
Carotid artery stenosis (blockage) has been successfully
treated for many years via a surgical method called carotid artery
endartecrectomy (CAE). But for patients unsuitable for surgical procedures,
carotid artery stenting (CAS) is a viable option, say the most recent
studies.
Reported by Burt Cohen, October 23, 2008
|



