|
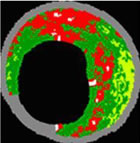
PROSPECT results presented today include the landmark finding that angiographically mild lesions with certain morphologic features on grayscale and VH IVUS present with a 3 year cardiac event rate of 17%, versus other morphologies (indistinguishable by conventional angiograms) with three year event risks of less than 1%. "For years, interventional cardiologists have been focused on using angiography to identify blockages and to restore flow. PROSPECT teaches us that looking at the angiographic severity of the blockage is not enough, and we must understand the type of disease present in the vessel wall," said Gregg Stone, MD, of Columbia University Medical Center and Principal Investigator of PROSPECT. "With VH® IVUS we can clearly see two things: one, that "mild" lesions on angiography are capable of progressing quickly into events for the patient: and two, events also take place in locations of severe disease that are often missed by angiography. Understanding the risk of each lesion type is paramount to minimizing the occurrence of these future events. Current practice using angiography guidance alone is insufficient in that regard." PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) is a first-of-its-kind multi-center, natural history study of acute coronary syndrome (ACS) patients. These patients underwent PCI to restore blood flow at baseline. Following the PCI procedure, an angiogram and a detailed IVUS image was captured in the three primary coronary arteries before the patient was sent home. If the patient had a subsequent event, they were readmitted, and again, an angiogram and IVUS imaging was performed. The core laboratory (Cardiovascular Research Foundation, New York, NY) then matched the location of the secondary event to the initial IVUS images to determine which locations progressed, and which lesions remained stable. Patient follow-up continued for three years. "Angiography alone is not enough and more and more clinical data continues to support that conclusion," said Scott Huennekens, President and CEO of Volcano. "PROSPECT has shown that not all coronary disease is the same. We cannot predict a patient's risk of having future cardiac events by looking at a 2-D, black-and-white image of the arterial lumen. Available tools like IVUS and VH® IVUS that look at the quantity, location and type of disease and not just the narrowing of the vessel improve our ability to determine the likelihood of a particular lesion type to cause an event. PROSPECT has shown the ability to use VH and grayscale IVUS to classify different lesion types per the PROSPECT definitions. This trial highlights not only the potential of Volcano's VH® IVUS, but just as important, the limitations of using angiography alone." VH® IVUS uses advanced spectral analysis of the ultrasound signal to classify atherosclerotic disease into different plaque components. By using these four plaque components, the PROSPECT investigators grouped the baseline lesions into five distinct lesion types; Fibrotic, Fibro-Calcific, Pathological Intimal Thickening, Thick-Cap Fibroatheroma and Thin-Cap Fibroatheroma, in order of hypothesized risk. PROSPECT ResultsThe PROSPECT event data presented today demonstrates that the measurements made using grayscale and VH® IVUS did have a statistically significant impact in determining the likelihood of a particular lesion type to cause an event. The highest risk PROSPECT plaque type being VH Thin Cap Fibroatheromas with a minimum lumen area of less than or equal to 4mm(2) and a plaque burden greater than or equal to 70%. All three of these important parameters (each an independent predictor) when combined showed that the particular lesion imaged at baseline had a 17.2% chance of causing an event within three years. It is important to note that current practice of using angiography alone cannot measure plaque burden or the presence of VH-TCFAs, which were the two most significant predictors of lesion risk in the study. These measures can only be collected using Volcano's proprietary Eagle Eye Gold IVUS catheter not only visualizes and measures plaque burden but also collects signals for VH imaging allowing display of four plaque components. Importantly, the absence of a fibroatheroma by PROSPECT definitions in imaged lesions was a strong predictor of lesion stability over three years as shown in the graph below. In lesions defined by PROSPECT having less than 10% Necrotic Core (red on VH IVUS), the likelihood of that lesion progressing to an event over three year was negligible, independent of other grayscale and angiographic factors. It is widely believed that a hazard ratio of greater than 2.0 is a clinically meaningful predictor of an event. You can see from the chart below that the hazard ratio for a PROSPECT defined VH-TCFA is 3.8 with a p-value of < 0.0001 indicating a positive predictive value of causing an event that is statistically significant. The converse is true for PIT lesions which have a hazard ratio of only 0.2, indicating low likelihood of causing an event, which is also statistically significant. "Healthcare reform has reached a boiling point," continued Huennekens. "The power of this study is quite simple: Angiography alone is not enough and we need more precise, targeted procedures. PROSPECT is not only important for identifying high-risk plaques so they can be treated to prevent future events. The findings are arguably just as important because they identify low-risk plaques, which do not need intervention which can decrease costs and future events. VH® IVUS is very different from non-invasive imaging technologies like CT and MRI, which have been accused of funneling too many procedures to the cath lab. In the case of IVUS, these patients are already in the cath lab, and are already getting an intervention. This tool simply allows physicians to further triage which lesions need to be stented, and which lesions can be treated with medical therapy. The negative predictive accuracy of VH® IVUS is very similar to Fractional Flow Reserve (FFR) in its potential to reduce procedure costs by stenting only the patients and lesions that will benefit from that therapy." Evening Symposium To explore the imaging data in greater detail, Volcano will host an evening symposium entitled, "Imaging Matters! Identifying high risk patients and optimizing PCI." The symposium will include late breaking results from PROSPECT, FAME and other clinical studies. The first session of the symposium, entitled, "Can imaging help identify at-risk vulnerable plaques and patients before catastrophe (or those not at risk)," will feature presentations from Patrick W. Serruys, M.D., Gary S. Mintz, M.D., Gregg W. Stone, M.D. and Alexandra Lansky, M.D. The second session, entitled, "Ischemia, FFR and Optimal Revascularization," will include presentations by Gregg W. Stone, M.D., Bernard de Bruyne, M.D., PhD, Bill Fearon, M.D. and Mort Kern, M.D. The event will be held at the Hilton San Francisco in the Imperial Room on Thursday, September 24. A reception will be held from 7:00 PM - 8:00 PM, followed by the symposium from 8:00 PM - 10:00 PM. About the PROSPECT Trial Abbott's PROSPECT trial is the first prospective natural history study to examine the role of vulnerable plaque and how it might progress to a cardiac event. PROSPECT used novel intravascular imaging technology to correlate plaque characteristics, patient risk factors and biomarker measurements with subsequent heart attacks and other cardiac events, potentially paving the way for physicians to identify and treat at-risk patients before a heart attack occurs. PROSPECT enrolled 700 patients from 40 clinical centers across the United States and Europe. All patients received PCI for acute coronary syndrome (ACS), which included unstable angina, NSTEMI or STEMI. Patient follow up continued for three years. Abbott sponsored the study and VH® IVUS imaging technology was provided by Volcano Corporation. About Volcano Corporation Volcano Corporation (NASDAQ: VOLC) offers a broad suite of devices designed to facilitate endovascular procedures, enhance the diagnosis of vascular and structural heart disease and guide optimal therapies. The company's intravascular ultrasound (IVUS) product line includes ultrasound consoles that can be integrated directly into virtually any modern cath lab. Volcano IVUS offers unique features, including both single-use phased array and rotational IVUS imaging catheters, and advanced functionality options, such as VH®) IVUS tissue characterization and ChromaFlo®. Volcano also provides functional measurement (FM) consoles and single-use pressure and flow guide wires and is developing a line of ultra-high resolution Optical Coherence Tomography (OCT) and Forward-Looking IVUS systems and catheters. Currently, more than 4,400 Volcano IVUS and FM systems are installed worldwide, and more than half of Volcano's revenues are derived from outside the United States. Through its wholly-owned subsidiary, Axsun Technologies, Volcano also develops and manufactures optical monitors, lasers and optical engines used in telecommunications, spectroscopy and other industrial applications. These products are sold to a variety of customers, including Nokia Siemens, Ericsson, Alcatel-Lucent and HuaWei Technologies. For more information, visit the company's website at http://www.volcanocorp.com. Forward-Looking Statements This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this release regarding Volcano's business that are not historical facts may be considered "forward-looking statements," including statements regarding the potential benefits of the procedures described above and of the Company's products, results and implications of the data from the PROSPECT trial and market adoption of the company's technology, the impact of clinical and other technical data. Forward-looking statements are based on management's current preliminary expectations and are subject to risks and uncertainties, which may cause Volcano's results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ from the results predicted are detailed in the company's annual report on Form 10-K, quarterly reports on Form 10-Q and other filings made with the Securities and Exchange Commission. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. Volcano undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events. Source: Volcano Corporation |