|
Catheter-Based Mitral Valve Repair Compares with Open Heart Surgery at One Year
|
 |
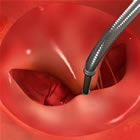
MitraClip® repairing
mitral
valve without surgery |
|
April 10, 2010 --
Atlanta, GA -- One of the more striking recent developments
in the surgery vs. angioplasty story
was presented at last month's American College of Cardiology
59th Annual
Scientific Sessions in Atlanta: The EVEREST II trial one
year results showed that a non-surgical approach to repairing
a leaky
mitral valve was successful and compared well with open heart
surgery, which is currently the gold standard of treatment
for mitral regurgitation (MR). Using a device delivered by
catheter,
snaked through
the femoral (groin) artery like coronary angioplasty
balloons and stents, cardiologists were able to repair mitral
valves successfully -- without surgery.
|
The device, originally developed by Menlo
Park-based eValve, Inc., is called the MitraClip® and was cleared
for use in Europe two years ago. In November 2009, Abbott acquired
eValve
and
the company
is hoping for a decision on FDA approval of the MitraClip sometime next year.
Results of the EVEREST II trial have been submitted to support that
process.
Mitral Regurgitation (MR)
Mitral
regurgitation is the most common type of heart valve insufficiency
and occurs when the leaflets of the mitral valve do not close completely,
causing
blood to flow backwards and leak into the left atrium of the heart
during the cardiac cycle. To maintain an adequate forward flow of
blood throughout the body, the heart compensates by increasing the
size of the left ventricle (main pumping chamber of the heart) to
accommodate the increase in the volume of blood it is pumping. When
this defect become significant, the condition becomes debilitating
and the heart's function deteriorates, leading to irregular
heartbeat, heart failure, stroke, heart attack or death. Mitral regurgitation
affects millions of people in the United States and Europe and
is currently managed with drugs or open heart
surgery,
depending
on an individual patient's severity of MR and risk factors. Some estimates place
the number of U.S. patients with significant MR at 250,000 -- yet
only 20% opt for repair, possibly because
the operation involves major open heart surgery.
EVEREST II One Year Results
The EVEREST (Endovascular Valve Edge-to-Edge Repair Study) II trial
enrolled 279 patients and is the first randomized clinical trial
to compare a percutaneously delivered device directly with open surgery
for
repair of the mitral valve. The measures were safety at 30 days and
efficacy at one-year.
At 30 days, the safety superiority over open
surgery was striking: 57% of the surgical patients experienced a
Major Adverse Event (defined as death, major stroke, reoperating,
urgent or emergency surgery, heart attack, kidney
failure, blood transfusions,
etc.) compared to only 9.6% of the MitraClip patients. Many of these
events were related to the need for blood transfusions, a point brought
up by surgeons at the presentation. However, as Dr. Ted Feldman of
Evanston Hospital in Illinois noted, there were no deaths, strokes
or emergency surgeries in the MitraClip cohort.
The one-year efficacy results
favored the surgical group of patients but, as defined by the
trial endpoints, the MitraClip was still "non-inferior".
The clinical success rate (freedom from death, surgery for valve
dysfunction or moderate to severe MR -- a grading of more than
2+) was 87.8% for the surgical group and 72.4% for the clip patients.
Although surgeons stated that they would not be comfortable if
their surgical
procedures resulted
in an MR grade of 2+ -- considered acceptable for the endpoint
in EVEREST II -- Dr. Feldman once again stated that patients
in the trial reported a significant increase in their quality
of life -- he commented:
"The most striking thing in our experience
is the remarkable clinical response. I've had patients who
literally could not walk across the road without getting
shortness of breath
and who go home after this procedure and, in less than a
week, are doing water aerobics."
|
|

Dr. Ted
Feldman
Evanston Hospital
Illinois |
What This May Mean for Patients
Although still not an approved device in the U.S., and perhaps not
as effective as open surgery, the potential usefulness of the MitraClip
is significant.
For example, many patients suffering from MR may be too ill or
elderly to be able to withstand the rigors of open heart surgery.
So certainly for
this
population, the minimally invasive nature of this catheter-based
procedure may
offer relief
from the debilitating effects of mitral regurgitation -- and may
slow down the onset of heart failure.
One point that was debated at the presentation was the issue of
being able to do future surgical repair on the mitral valve if,
after a number of years, the clip did not provide the necessary relief.
Dr. Craig Smith of Columbia University, in comments to theheart.org,
stated that he had done such a procedure a few years ago and found
the clip and surrounding tissue to be clogged and scarred and not
viable
for
surgical
repair. Whether this proves to be a problem for other cases is something
only "time will tell", he stated.
The MitraClip, along with other catheter-based
valve repair and replacement devices, such as the CoreValve from
Medtronic and the
Sapien from Edwards, represents the first wave of minimally invasive
structural heart interventions that ultimately may impact the field
of open heart surgery, much as the balloon and stent affected bypass
graft surgery.
Reported by Burt Cohen, April 10,
2010
|


