|
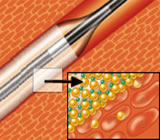
During TCT’s Drug-Eluting Stent (DES) Summit session on “Next Generation DES, Bioabsorbable Stents, and Drug-Eluting Balloons,” interventional cardiologist Bruno Scheller, M.D., professor of internal medicine and cardiology at the University of Saarland in Homburg/Saar, Germany, presented six-month final results of the investigator-initiated IN.PACT CORO ISR study on coronary in-stent restenosis. Prof. Scheller also presented an overview of the FreePac technology and interim findings from the larger IN.PACT clinical program, which includes studies of both coronary and peripheral applications of the IN.PACT family of drug-eluting balloons. “Based on a growing body of clinical data and while results of randomized trials are awaited, IN.PACT drug-eluting balloons show great promise for the treatment of both coronary and peripheral arterial disease,” Prof. Scheller said. “The best potential applications appear to be in atherosclerotic leg vessels and for previously stented coronary arteries that have restenosed. Drug-eluting balloons may very well become the fourth therapeutic platform for cardiovascular interventions, joining traditional balloon angioplasty, bare-metal stents and drug-eluting stents.” IN.PACT drug-eluting balloons feature a proprietary, hydrophilic coating called FreePac that frees and separates paclitaxel molecules, facilitating their absorption into the vessel wall to mitigate re-narrowing of the artery over time. The IN.PACT clinical program consists of studies on the treatment of de-novo coronary lesions and coronary in-stent restenosis, as well as below-the-knee (BTK) and superficial femoral artery (SFA) disease. A combination of company-sponsored and physician-initiated studies, it will enroll a total of approximately 1,500 patients. As part of his TCT presentation, Prof. Scheller highlighted IN.PACT CORO ISR, a single-center, single-arm confirmatory study of the IN.PACT Falcon DEB for the treatment of coronary in-stent restenosis conducted in 23 consecutive patients with a total of 26 lesions. The primary endpoint was late lumen loss measured by angiographic follow-up at six months. In the study, in-stent late lumen loss with the IN.PACT Falcon DEB was just 0.07 mm at six months, indicating a minimal degree of tissue growth inside the stented segment of the treated vessel. The newly-initiated IN.PACT SFA I trial is investigating the safety and efficacy of the IN.PACT Admiral paclitaxel-eluting percutaneous transluminal angioplasty (PTA) balloon catheter in the SFA compared to treatment with a standard PTA balloon. It will enroll 150 patients at up to 20 sites in Europe. The primary endpoint is clinically-driven target lesion revascularization (TLR, the need for repeat procedures) or restenosis (the re-clogging of an artery) at 12 months. Prof. Gunnar Tepe, M.D., chief of radiology at Klinikum Rosenheim in Germany, is the principal investigator. The first patient in the IN.PACT SFA I trial was treated earlier this month by Prof. Marianne Brodmann, M.D., and her team in the department of angiology at the University of Graz in Austria. Data from this trial may be combined with those from the IN.PACT SFA II trial, which will be performed in the United States, to file for approval by the U.S. Food and Drug Administration (FDA). The IN.PACT Admiral DEB and the IN.PACT Falcon DEB received the CE (Conformité Européenne) mark in 2009 and are available in more than 100 countries around the world. They are not available in Canada, Japan and the United States. Medtronic is committed to advancing the treatment of cardiovascular disease through collaboration with leading clinicians, researchers and scientists worldwide. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Source: Medtronic, Inc. |