|
Terumo Interventional
Systems First To Offer Multiple, All-In-One Radial Access Kits
For Double Or Single-Wall Entry Sticks
|
 |
 |
|
November 16, 2010 --
Somerset, New Jersey -- Terumo Interventional Systems, a strategic business
unit of Terumo Medical Corporation, and leading manufacturer
and distributor of guidewires, catheters, sheaths, and embolization
products, today announced the nationwide availability of the
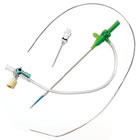
new Glidesheath™ Nitinol Kit, an all-in-one, micro-puncture
radial access kit for physicians who prefer transradial interventional
procedures. |
The new all-in-one Glidesheath Nitinol Kit expands
the product family portfolio and is available with unique features
such as a new .021” nitinol floppy palladium-tipped wire and a 21 gauge
metal needle. Unlike competitive kits that do not have a hydrophilic
sheath , each Glidesheath Nitinol Kit includes everything the operator
will need in one complete, convenient package.
"Terumo now offers a wide variety of specialized radial access kits to meet everyone’s needs for managing access from the radial artery. We are pleased to offer the new Glidesheath Nitinol Kit as an all-in-one solution for transradial procedures," said Gary Clifton, Senior Marketing Manager, Terumo Medical Corporation. "This
product expansion is another milestone in our commitment to delivering
a complete set of radial access tools that will enhance successful
outcomes and quality of life for patients."
Glidesheath Hydrophilic Coated Introducer Sheaths
offer the full-length Terumo Glide Technology™ coating to ensure smooth, atraumatic insertion and removal even in the most difficult procedures. The Glidesheath’s
unique design provides optimal flexibility for kink resistance to ensure
an open lumen throughout the procedure, while the cross-cut valve allows
for easy insertion of catheters and devices, while offering uncompromised
hemostasis.
Transradial access uses the arteries of the wrist, rather than the groin, to gain access to the heart. It is the leading access strategy in many countries of the world and while the technique accounts for an estimated three to five percent of cases in the U.S., there is a growing interest among physicians for more specialized tools that facilitate the procedure.
For more information about the new Glidesheath Nitinol Kit, please call 800-862-4143 to speak with an Inside Sales Specialist or visit www.terumois.com.
Terumo Interventional Systems
Terumo Interventional Systems (TIS), a strategic business unit of Terumo Medical Corporation, directly markets a full line of guidewires, catheters, introducer sheaths, guiding sheaths and embolization products for use in a multitude of different interventional procedures.
Interventional Radiologists, Interventional Neuroradiologists, Interventional Cardiologists, and Vascular Surgeons are among the medical professionals that depend upon TIS products to access and cross difficult-to-reach lesions, thereby allowing therapeutic intervention in previously unreachable vascular beds.
Terumo Medical Corporation
Founded in 1972 as a Terumo Corporation subsidiary, Terumo Medical Corporation (TMC) develops, manufactures, and markets high-quality medical devices used in a broad range of applications in numerous healthcare markets. TMC manufactures a broad portfolio of needles and syringes, entry-site management products, and a line of sterile connection devices used in hospitals and blood banks worldwide.
Terumo Corporation
Tokyo-based Terumo Corporation is one of the world’s leading medical
device manufacturers with $3.0 billion in sales and operations in more
than 160 nations. Founded in 1921, the company develops, manufactures,
and distributes world-class medical devices including products for
use in cardiothoracic surgery, interventional procedures, and transfusion
medicine; the company also manufactures a broad array of syringe and
hypodermic needle products for hospital and physician office use. Terumo
contributes to society by providing valued products and services to
the healthcare market and by responding to the needs of healthcare
providers and the people they serve.
Terumo Corporation’s shares are listed on the first section of the Tokyo Stock Exchange (No. 4543, Reuters symbol <4543.T>, or Bloomberg 4543: JP) and is a component of the Nikkei 225, Japan’s
leading stock index. Source: Terumo Interventional Systems
|

