
|
|
|
|
Medtronic Resolute® Drug-Eluting
Stent Delivers
Compelling Clinical Outcomes In Major Studies
Across Spectrum of Patients with Coronary Artery Disease, Novel Heart Device Shows Strong Results at One Year in RESOLUTE US and Two Years in RESOLUTE All Comers
|
 |
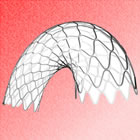
Medtronic's
RESOLUTE zotarolimus-eluting
coronary stent |
|
April 4, 2011 -- New
Orleans --Results of two major clinical studies presented
at ACC.11 – the
60th Annual Scientific Session & Expo of the American College
of Cardiology (ACC) – demonstrate that the Resolute® drug-eluting
stent (DES) from Medtronic, Inc. (NYSE: MDT), provides a positive
and persistent treatment effect for a wide variety of patients
with coronary artery disease.
One-year results of RESOLUTE US were
presented today in the late-breaking clinical trials session
for interventional cardiology and published by the Journal
of the American College of Cardiology (JACC). Two-year results
of
RESOLUTE All Comers were presented yesterday as a featured
interventional clinical study and published by The Lancet.
These studies are
key components of the comprehensive RESOLUTE clinical program,
which has enrolled more than 5,000 patients across a combination
of randomized controlled and single-arm trials conducted around
the world. |
These new data from RESOLUTE US complete Medtronic’s submission to the U.S. Food
and Drug Administration (FDA) for pre-market approval (PMA) of the Resolute DES,
which remains an investigational device in the United States, where its use is limited to
this FDA-approved clinical trial. The FDA’s decision is expected in the first
half of 2012.
"The latest findings from the uniquely designed RESOLUTE clinical program, with the
broad spectrum of patients enrolled, support this novel drug-eluting stent as a new
alternative in the treatment of coronary artery disease," said Martin B. Leon,
M.D., director of the Center for Interventional Vascular Therapy at NewYork-Presbyterian
Hospital/Columbia University Medical Center and a RESOLUTE US principal
investigator (PI). 'Based on the growing body of clinical evidence on the device,
the Resolute DES has distinguished itself as a viable choice for a wide variety
of patients."
The other PIs for RESOLUTE US are Laura Mauri,
M.D., chief scientific officer of the Harvard Clinical Research Institute
and an interventional cardiologist at Brigham and
Women’s Hospital in Boston, and Alan Yeung, M.D., director of interventional
cardiology at Stanford University School of Medicine in Palo Alto, Calif.
RESOLUTE US
One-year results of the 1,402-patient RESOLUTE US study include low rates of target
lesion failure (TLF, 4.7%), clinically-driven target lesion revascularization (TLR, 2.8%)
and definite/probable stent thrombosis (ST, 0.1%) through 12 months of follow-up.
These powerful clinical results were achieved despite 34% of the patients in the study
having diabetes, which typically correlates with higher event rates and represents an
underserved patient population.
RESOLUTE US is a prospective single-arm study
evaluating the safety and efficacy of the Resolute DES in a U.S. patient
population. It consists of four substudies based on
stent diameter and length: 2.25–3.5 mm Clinical, 2.25–3.5 mm Angio/IVUS, 4.0
mm Angio and 38 mm Clinical. The first three substudies of RESOLUTE US comprise
a
clinical cohort of 1,242 patients and an angiographic cohort of 160 patients.
All three have completed enrollment and have met their prespecified primary endpoints.
The 38
mm substudy is still enrolling patients.
The design of RESOLUTE US, with its four substudies,
will enable the FDA to evaluate the safety and efficacy of the entire
size matrix of the Resolute DES, from diameters of
2.25–4.0 mm and lengths of 8–38 mm. Total enrollment in RESOLUTE US will exceed
1,500 patients. The RESOLUTE clinical program will enroll a total of more than
5,000
patients worldwide.
RESOLUTE All Comers
Two-year results of the 2,292-patient RESOLUTE All Comers study indicate that the
Resolute DES continues to match the Xience V DES at two years of follow-up. On the
primary endpoint of TLF, originally assessed at one-year, the rates at two years remain
statistically equivalent for the two devices: 11.2% TLF for Resolute, 10.7% TLF for
Xience (p=0.736). In addition, the rates of definite/probable very late stent thrombosis
between one and two years were identical and low for each stent at 0.3%, with no
statistical difference between the two stents in cumulative ST through two years.
RESOLUTE All Comers is the first randomized
controlled trial to compare two second- generation drug-eluting stents
head-to-head: the Resolute DES and the Xience V DES.
(The Promus DES from Boston Scientific Corp. is identical to the Xience V DES.)
With
very few exclusion criteria, the study accepted virtually “all comers,” making the results
highly representative of routine clinical practice. Nearly 70% of the patients in
RESOLUTE All Comers were considered complex. By meeting the study’s primary non-
inferiority endpoint of TLF at one year, the Resolute DES was shown to match
the
Xience V DES on an important clinical outcome.
In collaboration with leading clinicians, researchers and scientists worldwide,
Medtronic offers the broadest range of innovative medical technology for the
interventional treatment of cardiovascular disease and cardiac arrhythmias.
About Medtronic
Medtronic, Inc. (www.medtronic.com),
headquartered in Minneapolis, is the global
leader in medical technology – alleviating pain, restoring health and extending
life for
millions of people around the world.
Any forward-looking statements are subject to
risks and uncertainties such as those
described in Medtronic’s periodic reports on file with the Securities and Exchange
Commission. Actual results may differ materially from anticipated results.
Source: Medtronic,
Inc.
|
|

