|
Upgraded ACCF/AHA/SCAI Guidelines Reinforce Benefits of Volcano's Precision Guided Therapy Technologies
|
 |
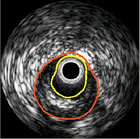 IVUS Image IVUS Image |
November 9, 2011 -- San Diego -- Volcano Corporation (NASDAQ: VOLC), a leading developer and manufacturer of precision guidance therapy tools designed to enhance the treatment of coronary and peripheral vascular disease, supports the elevation of Intravascular Ultrasound (IVUS) and Fractional Flow Reserve (FFR) technologies in the updated clinical guidelines for the management of patients undergoing percutaneous coronary intervention (PCI). The update was issued earlier this week by the American College of Cardiology Foundation (ACCF), American Heart Association (AHA), and Society for Cardiovascular Angiography and Interventions (SCAI).
"The latest guidelines recommend additional use of IVUS and FFR, both of which represent key product lines in Volcano's broad portfolio of precision guided technologies, in patients undergoing PCI," said Scott Huennekens, President and CEO of Volcano Corporation. "These recommendations confirm the positive role that IVUS and FFR technologies play in the care of PCI patients."
The newly revised guidelines include the use of IVUS for the assessment of intermediate lesions, for guiding stent implantation and for determining the cause of stent thrombosis. In addition, a new Class IIa recommendation now states that IVUS is reasonable for the assessment of angiographically indeterminate left main coronary artery disease (CAD), which is a new guideline and was not published in prior guidelines. IVUS and coronary angiography was also included for patients who have heart transplantation including 4-6 weeks post-cardiac transplant and 1 year follow up.
The guidelines categorize FFR as Class IIa and stated that the technology is reasonable to assess angiographic intermediate coronary lesions (50% to 70% diameter stenosis) and can be useful for guiding revascularization decisions in patients with stable ischemic heart disease.
The guidelines also included that every PCI program should participate in a regional or national PCI registry for the purpose of benchmarking its outcomes against current national norms. Technologies like IVUS and FFR help physicians make more informed clinical decisions and can have a positive impact on the quality scores of a healthcare institution.
About Volcano Corporation
Volcano Corporation is revolutionizing the medical device industry with a broad suite of technologies that make imaging and therapy simpler, more informative and less invasive. Our products empower physicians around the world with a new generation of analytical tools that deliver more meaningful information—using light and sound as the guiding elements. Founded in cardiovascular care and expanding into other specialties, Volcano is changing the assumption about what is possible in improving patient outcomes by combining imaging and therapy together. For more information, visit the company's website at www.volcanocorp.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this release that are not historical facts may be considered "forward-looking statements." Forward-looking statements are based on management's current expectations and are subject to risks and uncertainties which may cause Volcano's results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ include the pace and extent of market adoption of the company's products and technologies, growth strategies, timing and achievement of product development milestones, the impact and benefits of market development, product introductions, unexpected new data, safety and technical issues, market conditions, dependence on third parties, and other risks inherent to medical device development and commercialization. These and additional risks and uncertainties are more fully described in Volcano's filings made with the Securities and Exchange Commission, including our recent quarterly report on Form 10-Q. Undue reliance should not be placed on forward-looking statements which speak only as of the date they are made. Volcano undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
Source: Volcano
Corporation
|


