|
ADVISE: New Tool May Help Determine Stent and Angioplasty Appropriateness
ADVISE Study of instantaneous
wave-Free Ratio (iFR™) Published in JACC
|
 |
 |
December 7, 2011 -- ADVISE, a pilot study testing the validity of concept of a new adenosine-free assessment of stenosis severity, was published today in the Journal of the American College of Cardiology as an expedited manuscript. These encouraging results for a new measurement tool to help cardiologists decide when to place a stent seem timely, in light of the Centers for Medicare & Medicaid Services' (CMS) recent focus on the proper use of stents. Preliminary results of ADVISE had been previously presented at the TCT2011 meeting in San Francisco by Dr. Justin E. Davies of the National Heart and Lung Institute of Imperial College London.
Angioplasty.Org reported on this initial presentation and also posted an exclusive interview with Dr. Davies about this new procedure and its clinical implications, which may be significant.
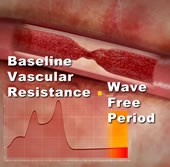
The study also provided a correlation of iFR against the current method for measuring intracoronary pressures, called fractional flow reserve (FFR). The r value in 157 stenoses measured was 0.90, virtual equivalence.
What is iFR, FFR and Functional Measurement?
FFR was developed over a decade ago by De Bruyne and Pijls as a more accurate way of determining the severity of a coronary blockage. And their FAME study, presented in 2009 (see our interview with Dr. Nico Pijls) showed that stenting, when guided by FFR instead of angiography alone, had better outcomes for patients. Instead of just looking at a blockage and judging it to be significant, FFR provided a way to actually measure whether or not the lesion was causing ischemia, a big question in intermediate blockages.
One drawback to FFR is that in order to accurately measure the pressure against the baseline resistance in the artery, a vasodilator drug, usually adenosine, must be administered during the procedure. This can cause some patient discomfort, extra time, and questions about dosing. However, Drs. Sayan Sen, Justin Davies and their team in London identified a short segment of the coronary waveform in which the resistance is stable (called the "wave free period") and, utilizing current computer sampling technology, they isolated that segment and were able to measure coronary pressure as accurately as FFR, without the need to administer any drug. Dr. Davies told Angioplasty.Org that an instantaneous measurement can be taken literally "by pressing a button".
Advantages of and Future Plans for iFR
The clinical implications of iFR are significant. As Dr. Morton J. Kern, Chief of Cardiology at Long Beach VA Hospital concluded in his accompanying editorial in JACC:

Morton J. Kern, MD |
"The iFR concept has great appeal. It would make lesion assessment quicker, easier, less expensive, and more widely used, but it must be carefully vetted before wholesale implementation."
Dr. Kern added in a conversation with Angioplasty.Org that a larger multicenter study is now underway and that, if validation of iFR is seen in that trial, then iFR might become available in a year or so. |
The instantaneous wave-Free Ratio (iFR™) technology was developed by the researchers at Imperial College in partnership with San Diego-based Volcano Corporation. Joe Burnett, Volcano's Executive VP and General Manager for Functional Measurement and Image Guided Therapy Business Units, commented to Angioplasty.Org on the results of ADVISE and a possible timeline for commercialization:
"In the environment that exists today, which requires physicians and hospitals to look beyond angiography toward additional tests to confirm appropriateness, we are very committed to making technologies like FFR and IVUS faster and easier than ever to fit into the workflow that interventionalists demand. Although we cannot comment on the timing of iFR™, as it is an investigational product, Volcano has made the development of this new measure of hemodynamic significance a top priority."
Role of Functional Measurement in Determining Appropriateness of Stenting
PCI appropriateness, mentioned above by Burnett, has become a hot topic in the past few months, amplified most recently by the CMS announcement of audits of certain procedures in 11 states to curb "improper payments". Disputes about over-stenting or unnecessary stenting have appeared in headlines repeatedly and disagreements whether or not a stent is needed in a particular situation have seen multiple panels looking at angiograms and second-guessing cardiologists' judgements.
Functional physiological measurement, such as iFR/FFR can provide additional information that's not possible to get from an angiogram alone. And Dr. Kern believes that this technology can resolve many of the issues that CMS is looking at. However, in the United States, the use of functional measurement is not fully reimbursed by CMS or other insurers, and thus is utilized in less than 10% of cases. Dr. Kern commented to Angioplasty.Org:
"If they're not going to pay for an objective measure of ischemia, but demand it, we're going to be hard-pressed to get doctors to cooperate. It's a conundrum. But I think an objective measure of lesion severity, especially for intermediate lesions, should be required in the absence of other indicators of ischemia: a stress test, EKG changes or something else.
"Functional measurement is a Class 1a recommendation in the European guidelines; but it's only a IIa recommendation in our guidelines. It's evident that this is a problem given the number of hospitals that are being investigated for the use of unnecessary stent implantation. CMS is obviously sensitized to this, yet they don't want to acknowledge that this tool would promote good care by bumping the reimbursement a little bit. I know they're under pressure to keep reimbursement to nothing, but this isn't the right way to take care of people."
Dr. Kern concluded:
"I think it's a good time for physiology to take its role as a good clinical indicator for procedures."
Reported by Burt Cohen, December 7, 2011
(Disclosures: Dr. Kern is a speaker for Volcano Corporation and St. Jude Medical, both of whom manufacture FFR equipment; Angioplasty.Org has received unrestricted educational grants from Volcano Corporation)
|



