|
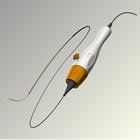
The model also projected that, over 10 years, renal denervation plus Standard of Care (SoC), treatment with three or more anti-hypertensive medications, could reduce cardiovascular mortality by 30 percent and all-cause mortality by 15 percent compared to SoC alone. Renal denervation with the Symplicity system was projected to substantially reduce 10-year and lifetime probabilities of stroke, myocardial infarction (MI), coronary heart disease (CHD), heart failure and end-stage renal disease (ESRD). "These results suggest that renal denervation with the Symplicity system is a cost-effective treatment strategy for resistant hypertension at a value substantially lower than the commonly accepted threshold," said Brent Egan, M.D. study co-author, professor, department of medicine, Medical University of Southern Carolina (MUSC). "Moreover, this health-economic model indicates that renal denervation with the Symplicity system may decrease mortality and reduce cardiovascular events in treatment-resistant patients, which would offer a major advancement in our approach to addressing this growing and costly disease." The model also found that median survival rates were estimated as 18.4 years for renal denervation with the Symplicity system compared to 17.1 years for SoC, and that cardiovascular end points might decrease by 21 percent to 32 percent over 10 years. Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. These nerves are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels. The Markov model used for this analysis was designed by Wing Tech Inc. to project the economic and clinical impact of renal denervation with the Symplicity system on patients with treatment-resistant hypertension. Treatment was defined as SoC plus catheter-based renal denervation with the Symplicity system. The estimated decrease in systolic blood pressure following renal denervation and other baseline patient characteristics were based on results of the Symplicity HTN-2 trial published in The Lancet in 20101. The characteristics of patients enrolled in the HTN-2 trial were mostly similar to the characteristics reported in other recent studies and registries of resistant hypertensives2,3,4, except for systolic blood pressure, as participants in Symplicity HTN-2 had to have a baseline systolic blood pressure of ³160 mm Hg per inclusion criteria. All other input parameters used in the model were derived from systematic searches of the literature. Cardiovascular event probabilities were obtained from the Framingham risk equations, except for the incidence of myocardial infarction for which the Prospective Cardiovascular Münster Heart Study (PROCAM) risk equation was used. End-stage renal disease incidence was estimated from the results of a more recent cohort study. Mortality rates were based on the most recent published estimates. "Our reliance on widely established multivariate risk equations, such as those from the Framingham heart study, allowed us to comprehensively assess and confirm the robustness of the model's projections across a wide range of cardiovascular risk profiles," said Jan B. Pietzsch, Ph.D., senior author and study director, president and CEO, Wing Tech Inc. Hypertension is the most common risk factor for the development of cardiovascular disease and leads to long-term cardiovascular and renal consequences that place a substantial burden on the health care system.5 Research suggests that nearly 30 percent of treated, uncontrolled hypertensive individuals are considered resistant to treatment, defined as having persistently high blood pressure despite three or more anti-hypertensive medications of different types.6 Treatment-resistant hypertension affects approximately 120 million people worldwide and is directly associated with increased risks of heart attacks, stroke, heart failure, kidney disease and death.6,7 These patients have a substantially increased risk of cardiovascular events compared to individuals with controlled high blood pressure.8 "As our clinical trial program and this cost-effectiveness analysis indicate, renal denervation with the Symplicity system represents an opportunity for Medtronic to help millions of patients worldwide while providing cost-effective solutions to our customers and healthcare systems," said Sean Salmon, Senior Vice President and President, Coronary & Renal Denervation, Medtronic. "We will continue to strengthen our leadership position in renal denervation therapy with additional research, including similar economic and clinical analyses in additional countries." About the Symplicity™ Renal Denervation System The Symplicity renal denervation system consists of a flexible catheter and proprietary generator. In an endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity™ catheter into the femoral artery in the upper thigh and threads it into the renal artery. Once the catheter tip is in place within the renal artery, the Symplicity™ generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm, or pattern, aiming to deactivate the surrounding renal nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant. The FDA granted Medtronic approval to conduct the Symplicity HTN-3 study, the company's U.S. clinical trial of the Symplicity renal denervation system for treatment resistant hypertension, in August 2011. Symplicity HTN-3 is a randomized controlled trial designed to evaluate the safety and effectiveness of renal denervation with the Symplicity renal denervation system in patients with treatment-resistant hypertension. The study will include approximately 530 treatment-resistant hypertension patients across up to 90 U.S. medical centers. More information about Symplicity HTN-3 can be found at www.symplifybptrial.com. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. ABOUT MEDTRONIC Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Source: Medtronic, Inc. |