|
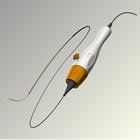
Treatment-resistant hypertension affects approximately 120 million people worldwide and is defined as persistently high blood pressure despite three or more anti-hypertensive medications of different types including a diuretic. Research suggests that nearly one third of treated hypertensive individuals are considered resistant to treatment1 and these patients have a three-fold increase in risk of cardiovascular events compared to individuals with controlled high blood pressure.2 "Persistent hypertension may increase the risk of stroke and cardiovascular events," said Dr. Kazuyuki Shimada, the former president of the Japanese Society of Hypertension, and principal investigator of the clinical trial. "This trial to confirm the efficacy of renal denervation in Japanese patients with the Medtronic renal denervation system may be the first step toward a positive future for patients with treatment-resistant hypertension, which can be difficult to treat with conventional blood pressure medications. If the results of the trial are positive, it may have a marked impact on the current strategies for treatment-resistant hypertension, for which effective approaches have not yet been established." Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. These nerves are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels. The Symplicity system's catheter and proprietary generator and algorithms were carefully developed through years of clinical experience to enhance the safety and effectiveness of the renal denervation procedure. The Symplicity renal denervation system has been used for five years to treat more than 5,000 patients with treatment-resistant hypertension worldwide. The Symplicity renal denervation system was launched commercially in April 2010 and is currently available in parts of Europe, Asia, Africa, Australia and the Americas. The Symplicity renal denervation system is not approved by the U.S. Food and Drug Administration (FDA) for commercial distribution in the United States or Japan. "The initiation of the Symplicity system clinical trial in Japan marks a pivotal milestone for the Symplicity clinical program and brings us one step closer to helping to address an unmet clinical need for people in Japan with treatment-resistant hypertension," said Sean Salmon, Senior Vice President and President, Coronary & Renal Denervation, Medtronic. "With the initiation of this study, we continue our broad commitment to partner with the global medical community to explore the use of renal denervation in different patient populations." About the Symplicity™ Renal Denervation System The FDA granted Medtronic approval for the protocol for SYMPLICITY HTN-3, the company's U.S. clinical trial of the Symplicity renal denervation system for treatment resistant hypertension in August 2011. More information about SYMPLICITY HTN-3 can be found at www.symplifybptrial.com. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. 1Egan, Brent M., et al. "Uncontrolled and Apparent Treatment Resistant Hypertension in the United States, 1988-2008." Circulation 124. 9 (2011): 1046-1058. 2 Doumas, Michael, et al. "Benefits from Treatment and Control of Patients with Resistant Hypertension." International Journal of Hypertension 2011 (2011) Article ID 318549, 8 pages, 2011. doi:10.4061/2011/318549. Source: Medtronic, Inc. |