|
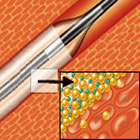
The largest randomized controlled trial of its kind, IN.PACT DEEP is designed to assess the safety and efficacy of the IN.PACT Amphirion drug-eluting balloon as a treatment for one of the most severe forms of peripheral artery disease –– critical limb ischemia that occurs below the knee. The result of an arterial obstruction in the leg, critical limb ischemia is a form of peripheral artery disease that significantly reduces blood flow to the lower extremities, causing severe rest pain that can lead to ulceration, gangrene and tissue loss. According to published data, 40 percent of critical limb ischemia patients undergo amputation within 12 months of an episode, and their annual mortality rate is more than 20 percent.1-2 IN.PACT DEEP enrolled 357 patients across 13 sites, randomizing them 2:1 to treatment with either the drug-eluting balloon (study arm) or conventional balloon angioplasty (control arm). Primary endpoints include late lumen loss and clinically-driven target lesion revascularization (TLR) at 12 months, as well as a composite of all-cause mortality, major amputation and clinically-driven TLR at six months. Other important endpoints, including wound healing and limb salvage rates, will also be examined and reported. The principal investigators of IN.PACT DEEP are Professors Iris Baumgartner of Bern University Hospital in Switzerland, Dierk Scheinert of the Heart Center in Leipzig, Germany and Thomas Zeller of the Heart Center in Bad Krozingen, Germany. "As the largest below-the-knee critical limb ischemia randomized trial, and the first and only drug eluting balloon multicenter randomized trial of any kind, IN.PACT DEEP has the potential to shift the treatment paradigm for this challenging patient population," said Prof. Baumgartner. "We look forward to sharing the initial safety, clinical and angiographic findings with the interventional community in 2013." With risk factors including a history of smoking, poor diet and lack of exercise (similar to those for other forms cardiovascular disease), peripheral artery disease affects more than 100 million people worldwide, including an estimated 10 million people in the United States, according to the Vascular Disease Foundation. Peripheral artery disease below the knee often causes critical limb ischemia, in which poor blood circulation in the calf, ankle, foot and toes can lead to ulcerated sores, amputation and premature death. Epidemiological research indicates that 50 percent of patients who undergo amputation due to peripheral artery disease die within five years. Medtronic's IN.PACT drug-eluting balloons feature a proprietary coating called FreePac that is a formulation of paclitaxel and urea, an excipient that facilitates absorption of the drug into the vessel wall. They received the CE (Conformité Européenne) mark in 2008 and 2009 and are available in many countries around the world. They are not commercially available in the United States. Ultimately, the global IN.PACT clinical program will include 29 studies involving more than 4,600 patients and 230 sites worldwide. Through these company-sponsored and physician-initiated studies, Medtronic IN.PACT drug-eluting balloons will be investigated thoroughly for the treatment of arterial disease in coronary and peripheral vessel beds. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Source: Medtronic, Inc. |