|
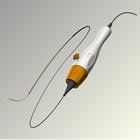
“These clinical trial results provide important evidence and confidence for physicians that renal denervation with the Symplicity system continues to be safe and effective in reducing blood pressure over time in patients with treatment-resistant hypertension, who are at high risk for various cardiovascular events,” said Markus Schlaich, M.D. F.A.H.A, professor of medicine and head of Hypertension & Kidney Disease at the, Baker IDI Heart and Diabetes Institute in Melbourne, Australia. “These data in nearly twice the number of patients previously reported at two years in this trial provide us with a better understanding of the patient response over time and add to the growing body of evidence supporting the safety and sustained blood pressure reduction of renal denervation treatment with the Symplicity system.” Responder rates (defined as a ³10 mm Hg reduction) among patients completing follow–up increased from 69 percent at one month to 82 percent at 24 months. Safety follow-up at 24 months demonstrated continued stable renal function with no new episodes of hypotension, orthostasis, or renal artery stenosis related to the renal denervation procedure; adverse events due to co-morbid diseases such as infection and non-renal surgical complications were reported. Pulse pressure improved significantly following treatment with the Symplicity system, with a reduction of -15 mm Hg [p<0.01] from baseline for the expanded patient cohort (n=105) at 24 months. Pulse pressure is the numeric difference between systolic and diastolic blood pressure and may have independent predictive value beyond absolute blood pressure measurements alone in terms of cardiovascular complications, especially in older patients. It may be important to evaluate changes in pulse pressure as well as systolic and diastolic blood pressure when assessing the efficacy of antihypertensive therapy. In this same analysis there were 34 patients available for follow-up at 36 months vs. 24 patients available at three years in the previously reported data. These 34 patients experienced a mean blood pressure reduction of -31/-16 mm Hg [p<0.01] at 36 months. For the patients in this analysis who were available for follow-up at 36 months there was a -16 mm Hg [p<0.01] reduction in pulse pressure following treatment with the Symplicity system. Responder rates among patients completing follow–up increased to 94 percent at 36 months. Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. These nerves are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels. The Symplicity system’s catheter and proprietary generator and algorithms were carefully and specifically developed through years of clinical experience to enhance the safety and effectiveness of the renal denervation procedure. The Symplicity renal denervation system has been used for five years to treat thousands of patients with treatment-resistant hypertension worldwide. The Symplicity renal denervation system is not approved by the U.S. Food and Drug Administration (FDA) for commercial distribution in the United States. About the SYMPLICTY HTN-1 Trial About Treatment-Resistant Hypertension About the Symplicity™ Renal Denervation System The Symplicity system consists of a flexible catheter and proprietary generator. In an endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity™ catheter into the femoral artery in the upper thigh and threads it into the renal artery. Once the catheter tip is in place within the renal artery, the Symplicity™ generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm, or pattern, aiming to deactivate the surrounding renal nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant. The FDA granted Medtronic approval for the protocol for SYMPLICITY HTN-3, the company’s U.S. clinical trial of the Symplicity renal denervation system for treatment resistant hypertension in August 2011. More information about HTN-3 can be found at www.symplifybptrial.com. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Symplicity is a trademark of Medtronic Inc. and is registered in one or more countries of the world. [1] Relative to medical management [2] No serious adverse events
Source: Medtronic, Inc. |