|
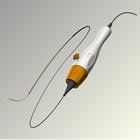
Also reported in the manuscript were 6 month results of 35 patients in the control group who received renal denervation after the primary endpoint was assessed at six months post randomization (referred to as the crossover group). The crossover group also showed a significant drop in blood pressure six months after the renal denervation procedure (-24/-8 mm Hg [p<0.001]). This decrease in blood pressure is similar to the blood pressure reduction in the initial treatment arm at 6 months. "We continue to see positive results from the Symplicity HTN-2 clinical trial, demonstrating consistent, long-term blood pressure reduction with the Symplicity system in patients with treatment-resistant hypertension, who have a three-fold increase in risk of cardiovascular events," said Murray Esler, M.B.B.S., Ph.D., principal investigator of the Symplicity HTN-2 trial and Senior Director of the Baker IDI Heart and Diabetes Institute of Melbourne, Australia. "These data further substantiate the clinical benefits of renal denervation with Symplicity over longer periods of time in this difficult-to-treat patient group." The Symplicity HTN-2 trial is an international, multi-center, prospective, randomized, controlled study of renal denervation in patients with treatment-resistant hypertension. One hundred and six (106) patients were randomly allocated in a one-to-one ratio to undergo renal denervation with previous anti-hypertensive medication treatment or to maintain previous anti-hypertensive medication treatment alone (control group) at 24 participating centers. Patients in the control arm of the study were offered renal denervation following assessment of the trial's primary endpoint at six months following randomization. Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. The nerves passing to the kidneys are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels. About Treatment-Resistant Hypertension About the Symplicity™ Renal Denervation System The Symplicity renal denervation system consists of a flexible catheter and proprietary generator. In an endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity™ catheter into the femoral artery in the upper thigh and threads it into both renal arteries in turn. Once the catheter tip is in place within the renal artery, the Symplicity™ generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm, or pattern, aiming to deactivate the surrounding renal nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant. The FDA granted Medtronic approval for the protocol for SYMPLICITY HTN-3, the company's U.S. clinical trial of the Symplicity renal denervation system for treatment resistant hypertension in August 2011. More information about HTN-3 can be found at www.symplifybptrial.com. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Symplicity is a trademark of Medtronic Inc. and is registered in one or more countries of the world. 1Egan, Brent M., et al. "Uncontrolled and Apparent Treatment Resistant Hypertension in the United States, 1988-2008." Circulation 124. 9 (2011): 1046-1058. Source: Medtronic, Inc. |