Today a spectrum of treatments are used for heart failure patients, ranging from medical therapy and lifestyle changes in lower risk patients to LVAD and heart transplantation in the highest risk. The annual cost of these surgical and non-surgical treatments, hospital visits and medications has been estimated at $56 billion annually in the United States alone. Hospital readmissions hover around 50% at six months, with mortality figures for hospitalized patients rising from a third at one year to half of those patients at five years.
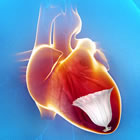
CardioKinetix states that with current medical therapy optimized to extent possible, advances in treatment of these patients will need to come from device-based solutions, such as the company's Parachute intravascular approach. In this procedure, dubbed "Percutaneous Ventricular Restoration (PVR)", the device is implanted in the left ventricle of a damaged heart and effectively walls off the non-functioning muscle, allowing the healthy portion of the heart to contract and expand more efficiently.
Data from the initial studies were presented this past year at EuroPCR and TCT. In the CE Mark approval study, 31 patients who received the Parachute device were tested at intervals up to three years for New York Heart Association functional classification. At baseline, 35% of these patients were Class I-II; by the end of the third year an impressive 87% could be included in this higher functioning cohort. The Class III portion was reduced from 65% at baseline to 14% of the patients at three years. Additionally the incidence of cardiac death remained steady at 6.5% from six months to three-years, demonstrating a "plateau" effect on left ventricular geometric changes.
Co-Principal Investigator, Marco A. Costa, MD, PhD, concluded his presentation at October's TCT meeting that, "the upcoming PARACHUTE IV large RCT will be critical to confirm or refute these promising results."
A press release from CardioKinetix announcing the start of the PARACHUTE IV Trial follows:
January 8, 2013
-- Menlo Park, California -- CardioKinetix Inc., a medical device company pioneering a catheter-based treatment for heart failure, announced the enrollment of the first patients in PARACHUTE IV, the randomized pivotal U.S. trial of the minimally invasive Parachute™ Ventricular Partitioning Device for the treatment of heart failure.
PARACHUTE IV is a multi-center pivotal trial designed to evaluate the PARACHUTE implant vs. optimal medical therapy (randomized 1:1) in approximately 500 patients with ischemic heart failure at up to 65 centers. The event-driven primary endpoint includes all-cause mortality and hospitalization for worsening heart failure. Other key endpoints include functional outcomes, quality of life, and hemodynamic measures by echocardiography.
The first implant with the Parachute device was performed by David Mego, M.D., assistant medical director at Arkansas Heart Hospital in Little Rock, Arkansas, who treated a 39-year-old female with NYHA class III heart failure referred by Carl Leding, M.D., director of the Congestive Heart Failure Clinic at Arkansas Heart Hospital.
Marco Costa, M.D., Ph.D., director of the Interventional Cardiovascular Center and the Research and Innovation Center at the Harrington Heart & Vascular Institute at Case Western Reserve University and co-principal investigator of the trial, said, "I am thrilled to be pioneering this exciting therapy in the United States, where there is a significant need for better treatments for this heart failure patient population."
"Many patients who have had a heart attack experience an extremely poor quality of life due to the debilitating symptoms of heart failure, including shortness of breath, fatigue, lack of appetite, impaired thinking, and increased heart rate. A classic example is the patient just treated at Arkansas Heart Hospital, where the patient experienced a heart attack less than two years ago and now struggles with simple things like dressing, cooking, or playing outside with her son because of shortness of breath and extreme fatigue," said William T. Abraham, M.D., the co-principal investigator of the trial and director of the Division of Cardiovascular Medicine and professor of internal medicine, physiology and cell biology at The Ohio State University Medical Center. "In October, three-year data on the initial patients treated with Parachute were presented at the TCT Conference by Dr. Marco Costa. These strong and encouraging results provide confidence to initiate a landmark randomized, pivotal trial where a positive outcome will establish this technology as a primary therapy option for the treatment of ischemic heart failure patients."
The Parachute device offers the first minimally invasive catheter-based treatment to partition the coronary muscle damaged by a heart attack. The treatment excludes the non-functional heart segment from the healthy, functional segment to decrease the overall volume of the left ventricle and restore its geometry and function.
"This initiation of the U.S. randomized pivotal study is a major milestone for CardioKinetix," said Maria Sainz, president and CEO of CardioKinetix. "We are excited about the opportunity before us to make a major positive impact for patients with heart failure, and we believe that we have partnered with the right heart failure physicians and interventional cardiologists to enroll this trial quickly."
About Heart Failure
Heart failure is a common, debilitating, and potentially deadly condition in which the heart is unable to supply sufficient blood flow to meet the needs of the body. Symptoms of heart failure negatively impact quality of life and include shortness of breath, persistent coughing or wheezing, buildup of excess fluid in body tissues (edema), fatigue, lack of appetite or nausea, impaired thinking, and increased heart rate. More than 20 million people around the world are affected, with approximately six million in the United States, where it is responsible for 1.1 million hospitalizations annually.[i]
About the Parachute™ Ventricular Partitioning Device
The first-of-its-kind Parachute Ventricular Partitioning Device is a minimally invasive treatment for patients with heart failure caused by damage to the heart muscle following a heart attack. Clinical data demonstrates improved overall cardiac function and quality of life for patients treated with the Parachute device.
Through a small catheter inserted in the femoral artery, the Parachute implant is deployed in the left ventricle to partition the damaged muscle, excluding the non-functional heart segment from the healthy, functional segment to decrease the overall volume of the left ventricle and restore its geometry and function. This minimally invasive procedure is performed in the catheterization laboratory under conscious sedation.
The Parachute Ventricular Partitioning Device received CE Mark in 2011. In the U.S., the Parachute system is an investigational device limited by federal law to investigational use only and is not available for sale.
About CardioKinetix Inc.
CardioKinetix, based in Menlo Park, Calif., is pioneering the catheter-based Parachute™ Ventricular Partitioning Device for heart failure. Privately held, the company is backed by SV Life Sciences, New Leaf Venture Partners, U.S. Venture Partners, JP Morgan Partners, and H&Q Healthcare Investors. For more information please visit www.cardiokinetix.com.