|
Medtronic's Drug-Eluting Balloon Data for Peripheral Angioplasty Submitted to FDA
First PMA Module Submitted; IN.PACT Admiral on Track to be First Drug-Eluting Balloon Approved for Use in the United States
|
 |
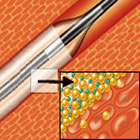
IN.PACT Drug-Eluting Balloon |
August 18, 2013 -- Medtronic, Inc. (NYSE: MDT) announced on Thursday that it had submitted its first pre-market approval (PMA) module to the U.S. Food and Drug Administration (FDA) for the IN.PACT Admiral drug-eluting balloon (DEB), to be used in the treatment of peripheral artery disease (PAD) in the superficial femoral artery. Although various IN.PACT drug-eluting balloons have been available in Europe for several years now, none are currently approved by the FDA for use in the United States.
Medtronic reports that with submission of this data, it is on track to gain FDA approval in two years, which would make it the first drug-eluting balloon available in the U.S. for use in the treatment of peripheral disease.
Angioplasty of the Leg: A Challenge
As reported this month in The Lancet, peripheral artery disease has now reached global pandemic proportions, having increased significantly in the previous decade, especially in low-to-middle-income countries.
Treating peripheral artery disease has proven to be one of the biggest challenges for the interventional cardiologist and radiologist. Compared to the coronary arteries, the arteries in the leg tend to be long and straight, and they aren't in a constant state of motion. However, once a blockage is opened, the peripheral artery has tended to reblock or restenose at a higher rate than the coronaries.
Various types of devices have been tried to alleviate this problems, from shavers (atherectomy devices) to drug-eluting stents, similar to those used in the heart. However, results from atherectomy vary, and stents can be subject to fracture, especially in parts of the leg that are subjected to compression and stress.
To date, the only drug-eluting stent approved for use in the United States has been the Zilver® PTX®, manufactured by Cook Medical. The Zilver PTX went through a recall in the Spring of 2013 for problems with separation of the catheter tip, but that issue has been addressed to the FDA's satisfaction and two weeks ago, the Zilver returned to the marketplace.
The concept of a drug-eluting balloon is attractive because such a device can be used in very narrow diameter vessels, and because no metal device is left in place to fracture or become the nexus for blood clots or excess tissue growth, eventually reblocking the vessel. Whether the DEB will be the solution to the problems encountered in treating peripheral artery disease will be seen as the multi-year results are measured. The IN.PACT clinical program is currently studying over 4,600 patients worldwide, looking at treatments in a variety of vessel locations.
Thursday's press release from Medtronic, Inc. follows:

Medtronic Submits First 'PMA' Module for IN.PACT Admiral Drug-Eluting Balloon
Med-Tech Leader Continues to Project Pre-Market Approval from
U.S. FDA for Novel Peripheral Angioplasty Device in Second Half of 2015
August 15, 2013 -- Minneapolis -- Marking a major milestone in the advancement of new treatments for peripheral artery disease, Medtronic, Inc. (NYSE: MDT) today announced the submission of its first pre-market approval (PMA) module to the U.S. Food and Drug Administration (FDA) for the IN.PACT Admiral drug-eluting balloon. Designed to treat atherosclerotic lesions in the superficial femoral artery (SFA), the novel angioplasty device remains investigational in the United States.
"Pending FDA approval, we remain on track to launch the IN.PACT Admiral drug-eluting balloon in the United States during the second half of calendar year 2015," said Tony Semedo, president of Medtronic's Endovascular Therapies business. "In the meantime, we will continue working with leading healthcare providers and researchers around the world to amass clinical and economic evidence to support the global adoption of this innovative medical technology as an important addition to the treatment options for peripheral artery disease in the lower extremities."
The ongoing global clinical program of IN.PACT drug-eluting balloons for the treatment of peripheral artery disease in the lower extremities includes 24 studies involving more than 4,200 patients at approximately 230 sites worldwide. A combination of physician-initiated and company-sponsored studies, the program will fully characterize the safety and efficacy of these combination devices in a variety of peripheral vascular beds, including below-the-knee arteries.
Medtronic's PMA application for the IN.PACT Admiral drug-eluting balloon will include clinical data from the IN.PACT SFA I and SFA II pivotal studies, the IN.PACT SFA II pharmacokinetics (PK) study, and the IN.PACT Global study, which have enrolled a total of more than 1,000 patients to date.
IN.PACT drug-eluting balloons feature a proprietary coating called FreePac that is a formulation of the antiproliferative drug paclitaxel and the biocompatible excipient urea, which facilitates rapid absorption of the drug into the vessel wall.
Several IN.PACT drug-eluting balloons received the CE (Conformité Européenne) mark in 2008 and 2009 and are available in many countries around the world. In the United States, the IN.PACT Admiral drug-eluting balloon is limited to investigational use under an investigational device exemption (IDE) granted by the FDA, and, like all drug-eluting balloons, is not yet commercially available.
The treatment of peripheral artery disease varies by stage of progression and other factors, with lifestyle modifications aimed at risk factor reduction recommended for all stages. Drug-eluting balloons represent a relatively recent addition to the procedural approaches, which also include atherectomy, balloon angioplasty and stenting. In cases involving especially long lesions, bypass surgery can create a new route for blood flow around the diseased artery.
With risk factors including smoking, diabetes, obesity, high blood pressure, high cholesterol, age (50 or older) and familial history, peripheral artery disease narrows the vessels that supply oxygenated blood to the body, especially the limbs. Typically characterized by an excessive buildup of plaque in these vessels, the condition can progress to claudication and, without treatment, to critical limb ischemia, which often leads to amputation and premature death. A growing global health concern, peripheral artery disease has increased in prevalence by nearly 24 percent worldwide over the most recent decade, from 164 million cases in 2000 to 202 million cases in 2010. [1]
In collaboration with leading clinicians, researchers and scientists, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers worldwide.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology-alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
[1] Prof F Gerald R Fowkes FRCPE, et al, Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis, The Lancet, Early Online Publication, 1 August 2013
Reported by Burt Cohen, August 18, 2013
|

