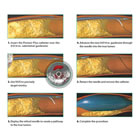
Intravascular Ultrasound (IVUS) is most commonly associated with use in the coronary arteries, to correctly size the native lumen of the artery and then to optimize placement of a stent, so that the entire area of disease can be covered, and also so the stent can be expanded to its optimal diameter. The use of IVUS has been associated with improved outcomes in angioplasty and stenting.
But the peripheral arteries present special challenges and, as reported this month in The Lancet, peripheral artery disease has now reached global pandemic proportions, having increased significantly in the previous decade. Clinical presentation of subtotal or total occlusions in the peripheral vessels presents further challenges, which can be addressed by the re-entry catheter procedure, shown in the graphic above. Through the use of this re-entry catheter technique, guided by intravascular ultrasound, completely blocked peripheral arteries can be opened up with an angioplasty balloon, possibly saving the patient from pain and possible amputation.
It is a full circle, since the concept of angioplasty was originated in the 60's by radiologist Dr. Charles Dotter, who developed concept of opening peripheral arteries from the inside out, instead of resorting to surgery. He would be amazed and delighted to see the sophistication of today's equipment.
Today's press release from Volcano Corp. follows:
Volcano Corporation Announces Execution Of Agreement To Purchase Pioneer Plus™ Re-Entry Catheter Product Line From Medtronic
Transaction Will Increase Company's Presence In Peripheral Indications And Expand Therapeutic Offerings
August 26, 2013 -- San Diego -- Volcano Corporation (Nasdaq: VOLC), a leading developer and manufacturer of precision guided therapy tools designed to enhance the diagnosis and treatment of coronary and peripheral vascular disease, said today it has signed an agreement to acquire the Pioneer Plus™ diagnostic ultrasound transducer and percutaneous catheter from Medtronic, Inc. (NYSE: MDT). The transaction is structured as an asset purchase and is expected to close by the end of August, subject to customary closing conditions.
The Pioneer Plus "re-entry" catheter, which has regulatory clearance in the U.S and Europe as well as other geographies, is an interventional medical device designed to enable the crossing of sub-total, total and chronic total occlusions (CTOs) within the peripheral vasculature, potentially eliminating the need for surgery. Volcano's IVUS (Intravascular Ultrasound) technology is used to direct the guidewire past stenotic lesions prior to additional interventions, such as balloon angioplasty or stent placement by providing a cross-sectional ultrasound image of the area of interest.

Scott Huennekens, President and CEO |
"We have been providing our IVUS technology in combination with the Pioneer Plus for many years," said Scott Huennekens, president and chief executive officer. "Being able to now offer this device within Volcano's already broad portfolio of products will serve our strategy of increasing our presence in the diagnosis and treatment of peripheral arterial disease (PAD). It also furthers our continued evolution from purely a diagnostic company to one that offers a wide range of diagnostic and therapeutic devices."
"With the completion of this transaction, the Pioneer Plus device will represent a further expansion of our peripheral therapeutics product line. At the end of 2012, we acquired the Crux Vena Cava Filter (VCF) System, a highly differentiated inferior vena cava (IVC) filter. We are readying a full market launch for the Crux device in the first half of 2014, while continuing our development program to combine our IVUS technology with the Crux VCF System."
"A key element of our growth strategy," Huennekens explained, "is to diversify beyond coronary applications and increase our penetration of the peripheral market. Over the past year, we have significantly expanded the presence of our imaging devices in the peripheral market. We have committed significant resources to this market through the creation of a dedicated sales force and expanded clinical research initiatives designed to increase utilization of both our IVUS and FFR (Fractional Flow Reserve) technologies for peripheral indications."
"The incidence of PAD is increasing globally and a significant number of treatment procedures require crossing stenotic lesions and CTOs," noted Neil Hattangadi, vice president and general manager, Peripheral Vascular Business Unit. "The Pioneer Plus catheter represents market leading technology that epitomizes the value of combining therapeutics with our IVUS technology."
"Clinical experience suggests that utilizing IVUS visualization with the Pioneer Plus aids in assessing the varying degrees of stenosis and may result in improved patient outcomes," Hattangadi continued. "We believe that adding the Pioneer Plus to our current portfolio of products will strengthen our sales and distribution efforts in the peripheral market, and will help facilitate further penetration of our diagnostic IVUS and FFR technologies in peripheral procedures. We currently offer our Valet® microcatheter and Eagle Eye® Platinum Short Tip IVUS catheter for use in highly stenosed peripheral lesions."
About Volcano Corporation
Volcano Corporation is revolutionizing the medical device industry with a broad suite of technologies that make imaging and therapy simpler, more informative and less invasive. Our products empower physicians around the world with a new generation of analytical tools that deliver more meaningful information—using light and sound as the guiding elements. Founded in cardiovascular care and expanding into other specialties, Volcano is changing the assumption about what is possible in improving patient outcomes by combining imaging and therapy together. For more information, visit the company's website at www.volcanocorp.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this press release regarding Volcano's business that are not historical facts may be considered "forward-looking statements," including: statements regarding the expected closing (including its timing) of the Pioneer acquisition; statements regarding Volcano's commercialization objectives, including planned product launches and the possible impact of the Pioneer acquisition on Volcano's sales and marketing efforts and penetration of the peripheral market; statements regarding the incidence of PAD and Volcano's strategy for increasing its presence in the diagnosis and treatment of PAD; statements regarding the design, capabilities, benefits (including clinical benefits) and value of the Pioneer device, both independently and in combination with Volcano's IVUS technology; statements regarding Volcano's growth strategy and corporate evolution and the ability of the Pioneer device to help Volcano achieve and promote that strategy and evolution. Forward-looking statements are based on management's current expectations and are subject to risks and uncertainties that may cause Volcano's results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ include the risk that the transaction described above is not completed, either at the anticipated time or at all; the risk that the benefits of the transaction described above (including as they relate to Volcano's commercialization objectives, growth strategy and corporate evolution and the capabilities, benefits and value of the Pioneer device) are not realized; the effect of competitive factors and Volcano's reactions to those factors; purchasing decisions with respect to Volcano's products; the pace and extent of market adoption of Volcano's products and technologies; uncertainty in the process of obtaining regulatory approval or clearance for Volcano's products and devices; the success of Volcano's growth strategies; risks associated with Volcano's international operations; timing and achievement of product development milestones; the outcome of ongoing and future litigation; the impact and benefits of market development; Volcano's ability to project its intellectual property; dependence upon third parties; unexpected new data, safety and technical issues; market conditions; and other risks inherent to medical device development and commercialization. These and additional risks and uncertainties are more fully described in Volcano's filings made with the Securities and Exchange Commission, including Volcano's most recent quarterly report on Form 10-Q, and other filings made with the SEC.
Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. Volcano disclaims any obligation to update any forward-looking statements to reflect new information, events or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.