|
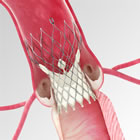
The FDA approved the CoreValve System without the need for an independent device advisory panel review due to its exceptionally positive clinical results demonstrated in the High Risk Study of the CoreValve U.S. Pivotal Trial. The head-to-head study, comparing transcatheter aortic valve replacement (TAVR) with the CoreValve System to traditional surgical aortic valve replacement, met its primary endpoint with high survival at one year for patients receiving the CoreValve System (85.8 percent), which was statistically superior to patients receiving a surgical valve (80.9 percent).
"This rigorous trial has defined a new standard for transcatheter valve performance, with superiority results that give physicians even more confidence in making TAVR treatment decisions," said David H. Adams, M.D., chair of the Department of Cardiothoracic Surgery at the Mount Sinai Hospital, New York City, national co-principal investigator of the CoreValve U. S. Pivotal Trial. "With this approval, we can treat more patients due to the broad range of CoreValve sizes, and we have an option compared to surgery that provides a greater chance for a longer life while minimizing the risk of stroke." For patients treated with the CoreValve System in the High Risk Study, rates of stroke - one of the most concerning complications of valve replacement because it increases the risk of death and can have a dramatic impact on quality of life - were low and not statistically different than rates experienced by surgery patients. The rate of MACCE (major adverse cardiovascular or cerebral events) was significantly better for CoreValve patients at one year, and overall hemodynamic (blood flow) performance was better in CoreValve patients than in surgical patients across all time points. The CoreValve System was designed to serve the clinical needs of the broadest range of patients with aortic stenosis. The valve's self-expanding frame provides controlled deployment, enabling physicians to accurately place the valve inside a patient's original valve, while conforming to the native annulus to provide a seal. The FDA approved the entire CoreValve platform - including the 23mm, 26mm, 29mm and 31mm size valves - all of which are delivered through the smallest commercially available TAVR delivery system (18Fr, or approximately 1/4 inch), making it possible to treat patients with difficult or small vasculature. "It's rewarding that we can now offer this life-saving therapy to patients at increased risk for surgery," said John Liddicoat, M.D., senior vice president, Medtronic, and president of the Medtronic Structural Heart Business. "There is a lot of excitement among U.S. heart teams for the CoreValve System's high risk approval, and its unique design that leads to the clinical outcomes seen in the High Risk Trial. We will continue to safely introduce CoreValve System to these physicians, supporting heart teams through comprehensive training and education, imaging and patient evaluation programs." Medtronic worked closely with the FDA throughout the pivotal clinical trial and Pre-Market Approval (PMA) review process. FDA granted Priority Review Designation for both the Extreme Risk and High Risk PMA submissions. Priority designation is granted to new therapies of major public health interest. Based on the strength of the trial data, FDA determined that sufficient information was available to evaluate the safety and efficacy of the Medtronic CoreValve System for both patient groups without the need for external Advisory Committee panels. This milestone, along with the priority review designation, accelerated regulatory approvals for the life-saving device. The CoreValve System was approved by the FDA for patients at extreme risk in January 2014. Since receiving CE (Conformité Européenne) Mark in 2007, the CoreValve System has been implanted in more than 60,000 patients in more than 60 countries. For more information about the CoreValve System, call 877-526-7890 or go to www.corevalve.com. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world. Multimedia Release About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Source: Medtronic, Inc. |