|
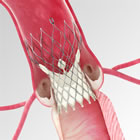
Two year results from 305 patients treated with the CoreValve System in the Extreme Risk Study of the CoreValve U.S. Pivotal Trial were presented during a late-breaking first report session at the Transcatheter Cardiovascular Therapeutics (TCT) 2014 Conference. Consistent with the positive one-year clinical results, all-cause mortality (36.5 percent) and major stroke (5.1 percent) at two years were low for this extreme risk cohort. The CoreValve device continued to demonstrate sustained favorable hemodynamic (blood flow) performance at two years as evidenced by low, single-digit mean gradients (blood flow resistance) of 8.7 mmHg; this is consistent with one month and one year rates (8.7 mmHg and 8.9 mmHg, respectively). Low rates of paravalvular leak (PVL) seen at one year were maintained, with just 4.4 percent of all treated patients experiencing moderate to severe PVL at two years. The study also found that the marked improvement patients experienced in heart failure symptoms at one year (as measured by NYHA Class) was maintained at two years: 92 percent of heart failure patients improved at least one class by two years, and 58 percent of patients improved at least two classes by two years. "The fact that nearly two out of three patients are alive at two years with low rates of stroke and re-hospitalization, and with substantial improvements in heart failure symptoms, is remarkable given patients' complex medical conditions, extreme frailty and inability to have a surgical procedure," said Steven Yakubov, M.D., who was an investigator in the CoreValve U.S. Pivotal Trial and is medical director at OhioHealth Research Foundation and system chief of Advanced Structural Heart Disease at OhioHealth. "In addition, the sustained hemodynamic performance of the valve gives us confidence in the durability of CoreValve therapy." The CoreValve System is approved by the U.S. Food and Drug Administration (FDA) for patients at extreme risk and high risk for surgery. Since receiving CE (Conformité Européenne) Mark in 2007, the CoreValve System has been implanted in more than 65,000 patients in more than 60 countries. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Source: Medtronic, Inc. |