|
FDA Approves HeartFlow FFRCT: Non-Invasive Method for Determining Coronary Ischemia
|
 |
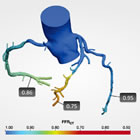
HeartFlow FFRCT 3D Analysis |
November 26, 2014 -- The U.S. Food and Drug Administration today approved HeartFlow's FFRCT software for measuring coronary blockages non-invasively. Fractional flow reserve
(FFR) has been validated through a number of clinical trials as a safe and effective means for measuring the extent of ischemia in the coronary arteries.
The DEFER, FAME and FAME 2 studies have shown that the stenting of blockages with an FFR higher than 0.80 can be safely deferred and treated with medical therapy alone.
The difficulty with FFR is that requires a pressure wire to cross the coronary lesion and can only be performed as part of an invasive cardiac catheterization.
The HeartFlow software is a ground-breaking technology that allows the data from a standard non-invasive Cardiac CT scan to yield measurements of ischemia, and at the same time show a 3D model of the arteries supplying blood to the heart. In this way, a single test can show (1) the extent and benefit of revascularizing the artery using angioplasty, stents, or possibly surgery. and (2) the precise anatomical characteristics of the coronary circulation, to aid the cardiologist or surgeon in a possible future intervention.

Dr. James K. Min |
Commenting on the importance of the FDA approval, Dr. James K. Min, Director of the Dalio Institute of Cardiovascular Imaging at NewYork-Presbyterian Hospital/Weill-Cornell Medical Center, told Angioplasty.Org:
"The FDA approval of the HeartFlow FFRCT technology is fantastic news to patients with suspected coronary artery disease and the physicians caring for these individuals. Based upon several prospective multicenter studies, FFRCT has been proven a disruptive technology that harnesses the power of computational fluid dynamics for diagnosis of physiologically-relevant coronary heart disease. Coupled with the anatomic information provided by coronary CT angiography, FFRCT for the first time facilitates a 'one stop shop' for discrimination of individuals who suffer from myocardial ischemia as well as the identification of the specific coronary artery lesions that are the cause."
The current accuracy of FFRCT to correctly differentiate significant blockages from those not needing revascularization is only in the mid-80% range. Yet the fact that a CT scan can be performed quickly, non-invasively, and with relatively low radiation doses, makes this procedure a significant contender to the extremely common nuclear stress test, and a potential game-changer in the diagnosis of coronary artery disease.
This morning's press release from the U.S. FDA follows:

FDA Allows Marketing of Non-Invasive Device to Help Evaluate Heart Blood Flow
November 26, 2014 -- Washington, DC -- The U.S. Food and Drug Administration today allowed marketing of the HeartFlow FFRCT software, which permits health care professionals to non-invasively evaluate blood flow in the coronary arteries of patients showing signs and symptoms of coronary artery disease.
Coronary artery disease, also called coronary heart disease, is the leading cause of death for both men and women in the United States. It occurs when one or more of the major arteries on the surface of the heart become narrow or blocked, reducing blood flow that supplies the heart muscle with oxygen-rich blood. Coronary artery disease can lead to chest pain (angina), heart attack, heart failure and death.
One piece of clinical information health care professionals use to determine the extent of a blockage in the heart or a coronary artery is a value called fractional flow reserve (FFR). Obtaining this value requires an invasive procedure called cardiac catheterization. The HeartFlow FFRCT software can non-invasively provide an estimate of FFR using data from a computed tomography (CT) scan of the patient's heart. The health care professional uses the estimate, along with other clinical patient data, to determine the likelihood that the actual FFR is below accepted limits and whether or not a more accurate FFR assessment using cardiac catheterization is necessary.
"HeartFlow FFRCT is a computer modeling program that provides a functional assessment of blood flow in the coronary arteries from detailed anatomical data," said William Maisel, M.D., M.P.H., deputy director for science and chief scientist in the FDA's Center for Devices and Radiological Health. "This non-invasive method is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures."
The HeartFlow FFRCT software is housed at HeartFlow, Inc.'s headquarters in Redwood City, California. A health care professional electronically sends the patient's CT scan data to HeartFlow, Inc. where a case analyst creates 3D computer models of different sections of the patient's heart and runs a blood flow simulator program on the models. After analyzing the data and the models, the case analyst electronically sends a report with the estimated FFR values (called FFRCT values) displayed as color images of the patient's heart.
The FDA reviewed the data for HeartFlow FFRCT through the de novo premarket review pathway, a regulatory pathway for some low- to moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.
Data submitted to support the safety and effectiveness of HeartFlow FFRCT included clinical studies that compared FFRCT measurements to FFR values directly measured by cardiac catheterization on subjects with suspected coronary artery disease who were therefore referred for catheterization and FFR. The results showed that Heart Flow FFRCT was able to correctly identify 84 percent of the significant blockages identified by FFR as requiring intervention, and 86 percent of blockages identified by FFR as not requiring intervention. The company also submitted data and information showing how they have mitigated risks associated with the device, such as controlling for erroneous calculations that can lead to delayed or improper treatment.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation's food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Reported by Burt Cohen, November 26, 2014 (updated 11-27-2014 with quote from Dr. Min)
|



