|
FDA Approves New Repositionable and Recapturable CoreValve from Medtronic
First and Only Repositionable and Recapturable TAVR Approved in U.S.
Evolut R's 14F Delivery System is Lowest Profile Available
|
 |
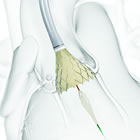
CoreValve® Evolut™ R |
June 23, 2015 -- Today the FDA approved Medtronic's (NYSE: MDT) next-generation CoreValve Evolut R System for severe aortic stenosis patients who are at high or extreme risk for surgery. The Evolut R is the first and only recapturable and repositionable TAVR system available in the United States.
A challenging aspect of TAVR procedures has always been obtaining optimal valve placement. Unlike valves placed during open surgery, the transcatheter valves are advanced through an artery to the correct location, and then deployed, using angiography as a guide. Until now, it has not been possible to easily move or "nudge" the valve to a slightly better position, if the initial placement was slightly off. Since the purpose of the TAVR device is to replace a poorly-functioning aortic valve, one which leaks, a proper fit is essential to better patient outcomes.
In addition, the Evolut R system is delivered through a 14F system, the narrowest profile currently available, and may reduce the type of vascular access complications that can occur with these larger devices (stents, for example, are usually placed with much thinner 6F systems). This thinner delivery profile will make it possible to treat an expanded patient population with TAVR, especially those with narrower vessels.
Although first developed a decade ago, use of the TAVR procedure has been growing rapidly, as indicated in this past weekend's New York Times feature by Gina Kolata, "Building a Better Valve." The Edwards Sapien valve and Medtronic's CoreValve were first approved in Europe in 2007. Four years later the Sapien was approved for use in the U.S. Medtronic's CoreValve was approved by the FDA in 2014, and since has been given expanded indications. Both valves started with indications only for patients judged too sick to withstand open surgery, and have since been widened to be appropriate for those at "high risk" for surgery. Earlier this year, Medtronic's CoreValve received approval for valve-in-valve procedures, where the TAVR could replace a failed surgical valve.
Of notable interest was a study presented at the 64th Annual Scientific Session of the American College of Cardiology (ACC.15) in March that showed the CoreValve to be superior to open surgery at two years in patients at high risk for surgery.
As these devices and the techniques for placing them continue to improve, it is possible that in the not-too-distant future, TAVR may become the preferred method of aortic valve replacement, even in patients at lower risk - a trend that has occurred in the repair of abdominal aortic aneurysms (AAA).
This morning's press release from Medtronic, Inc. follows:

Medtronic Announces FDA Approval for New TAVR System, Introduces First and Only Recapturable Heart Valve in U.S.
CoreValve® Evolut™ R Recapturable and Repositionable Heart Valve Improves Positioning Accuracy and Control During Deployment
June 23, 2015 -- Dublin -- Medtronic plc (NYSE: MDT) today announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the new recapturable, self-expanding CoreValve® Evolut™ R System. The first-and-only recapturable and repositionable device available in the U.S., the Evolut R System is approved for transcatheter aortic valve replacement (TAVR) in severe aortic stenosis patients who are at high or extreme risk for surgery. Untreated, aortic valve stenosis can lead to serious heart problems including heart failure and even death.
Designed to treat patients with aortic stenosis, a condition where the aortic valve narrows thereby limiting blood flow from the aorta to the rest of the body, the CoreValve Evolut R System is built on the proven foundation and procedural success of the CoreValve System, which has been implanted in more than 75,000 patients in 60 countries.
"In a short time, the TAVR procedure has become an established treatment option for high risk patients with severe aortic stenosis who are unable undergo surgery, and physicians are looking to technology advancements to help deliver even better patient outcomes," said Mathew Williams, M.D., co-primary investigator for the study, as well as chief of Adult Cardiac Surgery and director of Interventional Cardiology and Structural Heart at the NYU Langone Medical Center in New York City. "Clinical data have shown the best patient outcomes are achieved when the valve is properly positioned. The advancement of recapturability with Evolut R gives physicians more confidence during the procedure and provides advantages that are non-existent in other TAVR systems."
The new system consists of the CoreValve Evolut R transcatheter valve and the EnVeo™ R Delivery System, which features an InLine™ Sheath that significantly reduces the profile to the lowest on the market (14 Fr equivalent, less than 1/5 inch). A smaller profile size provides a greater opportunity to treat an expanded patient population with smaller vessels (down to 5.0 mm), through the preferred transfemoral access route, which may minimize the risk of major vascular complications in some patients.
Based on the knowledge gained through the extensive experience with the CoreValve System, the Evolut R is optimized to increase conformability and sealing at the annulus, while maintaining supra-annular valve positioning for improved blood flow and hemodynamic performance. An extended sealing skirt on the 26mm and 29mm valve sizes is intended to further promote valve sealing at the annulus.
"The FDA approval of Evolut R marks a significant milestone for Medtronic and TAVR, and ushers in a new era in transcatheter aortic valves with advanced, recapturable capabilities," said Rhonda Robb, vice president and general manager, Heart Valve Therapies, Medtronic. "This approval is an outcome of our commitment to building a market-leading innovation pipeline in the transcatheter space, and we look forward to supporting heart teams as they look to next-generation technologies that optimize valve performance for a broad range of patients."
The approval of Evolut R as the first self-expanding, recapturable transcatheter heart valve available in the U.S. follows other significant milestones reached in recent months showcasing the company's leadership in the TAVR market. In March, the CoreValve System was the first TAVR system to be approved in the U.S. for valve-in-valve (VIV) procedures in patients whose surgical aortic heart valves have failed. Also in March, the highly anticipated two-year data from the High Risk Study of the CoreValve U.S. Pivotal Trial was presented at ACC.15, which showed superior survival benefit at two years for TAVR with the CoreValve System compared to patients who underwent surgical aortic valve replacement (SAVR).
The 23 mm, 26 mm and 29 mm sizes of the CoreValve Evolut R transcatheter valve and the CoreValve EnVeo R Delivery Catheter System are available for use in the United States. The device is also available in Europe and other countries that recognize the CE (Conformité Européene) mark.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is the global leader in medical technology - alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Reported by Burt Cohen, June 23, 2015 |

