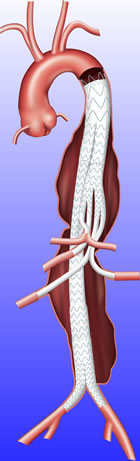
Thoracoabdominal aortic aneurysms are a rare, complex and deadly condition that affects the largest artery in the body, the aorta. The only treatment option for TAAA is open surgery, which is associated with a 25% mortality rate.
Sanford Health’s Dr. Patrick Kelly performed the first procedure on February 9 and is leading the PSIDE study to address this unmet clinical need to and improve the health of patients suffering with this complex aortic disease.
It was only 25 years ago that the first AAA stent graft procedure was performed in Buenos Aires, Argentina by Dr. Juan Parodi. At the time Dr. Parodi was "pilloried" by the medical establishment, in the words of Dr. Hugh Beebe. There was intense skepticism as to the viability of repairing an aortic aneurysm from the inside-out instead of with open surgery.
Today AAA repair is routinely done using Parodi's endovascular method. The endovascular approach to TAAA repair, being tested in this current trial, represents the latest advance in this relatively new non-surgical approach to vascular therapy.
The history of this transition, from open surgery to endovascular repair, is portrayed in Angioplasty.Org's documentary, "Vascular Pioneers: Evolution of a Specialty." Spoiler Alert: what used to be done with traditional open surgery is now being done more and more using minimally invasive therapy, Vascular surgeons now use catheters and wires instead of the scalpel.
First Patient Treated with Medtronic Valiant TAAA Stent Graft System in Thoracoabdominal Aortic Aneurysm Study
Sanford Health in Collaboration with Medtronic Leads Global Efforts to Address Challenging Vascular Condition
February 9, 2016 -- Dublin and Sioux Falls, South Dakota -- Medtronic plc (NYSE:MDT), a global leader in medical technology, services and solutions and Sanford Health, one of the nation's largest health care systems, today announced the first patient enrolled in a clinical study using the Medtronic Valiant® TAAA Stent Graft System for minimally invasive repair of thoracoabdominal aortic aneurysm.
An aortic aneurysm is a dangerous bulge or ballooning in a segment of the wall of the aorta that can rupture and cause sudden death if left untreated. Thoracoabdominal aortic aneurysms start in the chest and extend through the abdomen, and represent about 15 percent of all thoracic aneurysms. The aneurysms typically involve the branch arteries that supply blood to multiple internal organs. Historically this challenging anatomy has been treated with a complex open surgical procedure that is associated with high morbidity and mortality.
The procedure was performed by Patrick Kelly, M.D., through a physician sponsored, investigational device exemption (PS-IDE - NCT02294435), approved by the U.S. Food and Drug Administration (FDA). Dr. Kelly, a physician and inventor who leads Sanford Vascular Innovations, reports the patient was a 58-year-old female with few alternative medical options for treatment of her aneurysm.
"The mortality rate is 25 percent when treating a thoracoabdominal aortic aneurysm with an open surgical technique, which involves cutting open the aorta. Providing the patient with an option for a less-invasive approach is needed," said Dr. Kelly. "This procedure marked an important step in the process to obtain FDA approval, and Sanford's support of such innovation will give hope to patients afflicted with challenging disease states such as this."
The Valiant TAAA Stent Graft System is intended to allow for an off-the-shelf endovascular solution to one of surgery's most difficult pathologies. The device garners broad attention as physicians search for options to treat patients with severe and challenging aortic disease.
"The Valiant TAAA approach allows for the procedure to be staged at any time and lets the operator work on each branch vessel individually," said James Black III, M.D., chief of the Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Hospital. "When taking on challenging cases, the device leverages skill sets that are quite routine for vascular surgeons."
"The novel device is customizable and diverse," said Thomas Naslund, M.D., chief of the Division of Vascular Surgery at Vanderbilt University Medical Center. "It's critical that our field focus on innovative ways to treat these kinds of aneurysms."
"As the first patient treated with the minimally invasive Valiant TAAA recovers, we are inspired by the innovators that continue to challenge the limitations of current treatment options," said Daveen Chopra, vice president and general manager of the Aortic business, which is part of the Aortic & Peripheral Vascular division at Medtronic. "Our mission to improve patient outcomes will drive us into the future as we seek to treat more complex aortic disease."
A concept for the novel Valiant TAAA system was first described in the November 2014 issue of the Journal of Vascular Surgery. Dr. Kelly developed the concept for the system and has since collaborated with Medtronic. Sanford Health holds the intellectual property covered by the exclusive patent license agreement with Medtronic. Medtronic plans to study the system in collaboration with physicians at several medical centers, including Dr. Kelly at Sanford Health.
Medtronic is the long-standing leader in medical technology for endovascular aortic repair. Nearly one in every two endovascular aortic repairs performed worldwide are done with a Medtronic product.
About Sanford Health
Sanford Health is an integrated health system headquartered in the Dakotas. It is one of the largest health systems in the nation with 43 hospitals and nearly 250 clinics in nine states and four countries. Sanford Health's 27,000 employees, including 1,400 physicians, make it the largest employer in the Dakotas. Nearly $1 billion in gifts from philanthropist Denny Sanford have allowed for several initiatives, including global children's clinics, genomic medicine and specialized centers researching cures for type 1 diabetes, breast cancer and other diseases. For more information, visit sanfordhealth.org.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies - alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 85,000 people worldwide, serving physicians, hospitals and patients in approximately 160 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.