|
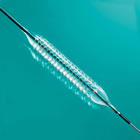
Absorb is the only fully dissolving stent approved for the treatment of coronary artery disease, which affects 15 million people in the United States and remains a leading cause of death worldwide, despite decades of therapeutic advances. While stents are traditionally made of metal, Abbott's Absorb stent is made of a naturally dissolving material, similar to dissolving sutures. Absorb disappears completely[1] in approximately three years, after it has done its job of keeping a clogged artery open and promoting healing of the treated artery segment. By contrast, metal stents are permanent implants that restrict vessel motion for the life of the person treated.
"The Absorb bioresorbable scaffold represents a major advance in the treatment of coronary artery disease," said Gregg W. Stone, M.D., FACC, FSCAI, director, cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia University Medical Center, New York-Presbyterian Hospital and the chairman of the ABSORB clinical trial program. "This novel technology appeals to both physicians and patients alike because after treating the underlying blockage it is completely absorbed, leaving nothing behind. No metal means the treated artery can pulse and flex naturally as demands on the heart change with everyday activities. No metal may also reduce the potential of future blockages that occur with permanent metallic stents, and allows easier access to other treatment options should they prove necessary in the patient's future." Abbott plans to offer the Absorb device to hospitals in the United States, starting with interventional cardiology centers that participated in Absorb clinical trials. "Abbott's goal is to help people everywhere live better, fuller and healthier lives," said Deepak Nath, Ph.D., senior vice president, vascular, Abbott. "The Absorb bioresorbable stent treats coronary artery disease without committing people to a permanent metal implant—giving them peace of mind and helping them get back to their daily lives without the concern of having a permanent metallic implant. We're very excited to bring the promise of Absorb to patients in the United States." In clinical studies conducted around the world, the Absorb bioresorbable stent demonstrated comparable short-term and mid-term outcomes to the leading metallic stent—Abbott's Xience™ drug eluting stent. At one year in a pre-specified group of approximately 2,000 U.S. patients in the pivotal ABSORB III randomized clinical trial, patients who received the dissolving Absorb stent experienced comparable rates of specific adverse events in the intended patient population (reference vessel diameter of > 2.5 mm and < 3.75 mm)—including heart disease-related death, heart attacks attributed to the stented artery and repeat procedures at the treated lesion (collectively termed target lesion failure)—as compared to patients who received the metallic Xience stent. Abbott's Absorb stent, sold commercially as the Absorb GT1 Bioresorbable Vascular Scaffold (BVS) system, is now available in more than 100 countries, including the United States, and has been used to treat more than 150,000[2] people with coronary artery disease worldwide. For more information on Absorb, including important patient safety information, please visit www.dissolvingstent.com. About Abbott Connect with us at www.abbott.com, on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews and @AbbottGlobal. Source: Abbott |