|
Boston Scientific Issues Urgent Lotus Valve Recall
Global Recall for All Devices Manufactured Prior to March 2016
Malfunction in Delivery System Only: Performance of Implanted Devices Not Affected
Release Mandrel Breaks in Delivery System Resulted in Three Patient Deaths
|
 |
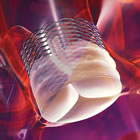
Boston Scientific's Lotus Valve for TAVR |
August 3, 2016 -- Boston Scientific has issued a global recall of all Lotus™ transcatheter aortic valves that were manufactured before March 2016.
According to a Field Safety Notice issued by Boston Scientific International S.A. on August 2, only the products with the following Material Numbers (UPN) are involved: H749LTV230, H749LTV250, and H749LTV270.
The specific Lot Numbers are listed in the Field Safety Notice.
The problem with the Lotus Valve was not with the valve itself, but with a component of the delivery system. A manufacturing change was made to this component in March 2016. According to Boston Scientific:
"This action is related to a release mandrel breaks. The release mandrel is a component of the delivery system that is connected to the release pin which facilitates release of the valve from the delivery system. There were a number of release mandrel breaks reported for product manufactured prior to the component change. The most common outcome was resheathing
and removing the device without difficulty, resulting in procedural prolongation. However, the most severe outcome was catastrophic vessel trauma associated with patient death, which was reported in three cases."
The manufacturing change to the release mandrel was made in March 2016 and no issues have been reported in subsequent products. The Company stresses that the flaw is only in the delivery system of Lotus Valves manufactured prior to March 2016. The performance of all valves that are currently implanted in patients, whether manufactured before or after March 2016, is not affected, since the issue is with the delivery system only. No reports of release mandrel breaks have been received since the component change. The Company emphasizes that "there is no impact to previously implanted devices."
The Company did not state when reports of the three patient deaths were received, but it is assumed they were recent, leading to the urgent recall notification.
Information for hospitals on how to proceed returning the affected products is detailed in the Field Safety Notice.
Reported by Burt Cohen, August 3, 2016
|

