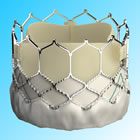
This approval represents a major advance for the non-surgical minimally invasive treatment of aortic stenosis. Data from the PARTNER II clinical trial that was presented in April at the annual American College of Cardiology meeting, and published simultaneously in The Lancet, showed that in these intermediate risk patients, the transcatheter TAVR approach was not only equivalent to open surgical valve replacement, but it was superior. Mortality at one year was almost halved in those patients treated with TAVR.
Of interest is the fact that the SAPIEN3 has not yet been approved for intermediate risk patients by the Conformité Européene (the CE Mark). This is an unusual situation because FDA approval almost always comes well after the CE Mark is awarded. For example, Medtronic's repositionable TAVR device, the CoreValve Evolut R, received the CE Mark for use in intermediate risk patients earlier this month, but may have to wait for an FDA approval until later this year.
Reaction among the inteventional cardiology community was quick. Dr. Jonathan Reiner of George Washington Hospital tweeted: "With S3 now approved for intermediate risk patients (possibly the largest pool) TAVR is quickly becoming the default strategy for AVR" and added, "With TAVR rapidly expanding, CT surgeons must decide whether to embrace (and train for endovascular) or fight like the old lost PCI wars." Dr. Chandan Devireddy of Emory Hospital exclaimed, " Finally! @FDADeviceInfo approves SAPIEN 3 transcatheter valve #TAVR for intermediate risk patients." And Dr. Jordan Safirstein of Morristown Medical Center opined, "Let the flood gates open!"
Indeed, FDA approval for intermediate risk patients opens the market for this device considerably. This approval also validates the transcatheter approach to the treatment of heart disease envisioned almost 40 years ago by Andreas Gruentzig, who performed the first coronary angioplasty. He stated: "Whatever becomes of the method, I have left one mark on medicine: I have shown that man can work therapeutically within the coronary arteries themselves in the face of an alert, comfortable patient."
Edwards SAPIEN 3 Transcatheter Heart Valve Receives Expanded Indication From FDA
August 18, 2016 -- Irvine, California -- Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients suffering from severe, symptomatic aortic stenosis who have been determined by a Heart Team to be at intermediate risk for open-heart surgery. The SAPIEN 3 valve is the first transcatheter aortic valve replacement (TAVR) therapy to obtain this indication in the United States.

Dr. Vinod Thourani |
"The SAPIEN 3 valve has set a new standard for performance and patient outcomes with aortic valve replacement," said Vinod Thourani, M.D., co-director of Emory Heart and Vascular Center's Structural Heart and Valve Center and professor of cardiothoracic surgery at Emory University School of Medicine. "The clinical outcomes of 1.1 percent mortality and 1.0 percent disabling stroke at 30 days in this intermediate-risk population treated with the SAPIEN 3 valve are changing the paradigm of how we treat patients with aortic stenosis."
Thourani is the co-principal investigator of the SAPIEN 3 valve study.
"The intermediate-risk approval of the SAPIEN 3 valve is a major milestone, since it provides a less-invasive therapy that has demonstrated better outcomes for aortic valve patients, and is supported by the largest and rigorous comparative body of evidence for the treatment of aortic stenosis," said Larry L. Wood, Edwards' corporate vice president, transcatheter heart valves.
The SAPIEN 3 valve intermediate-risk approval was based on data from a cohort of the PARTNER II Trial, which studied 2,005 intermediate-risk patients at 51 sites in the United States and Canada. The study demonstrated that patients treated with the SAPIEN 3 valve experienced clinically significant improvements for the composite primary endpoint of mortality, stroke and moderate or severe aortic regurgitation at one year as compared to those treated with surgery. The data were presented in April at the American College of Cardiology's 65th Annual Scientific Session and simultaneously published in The Lancet.
The expanded intermediate-risk indication granted by the FDA enables Heart Teams to treat patients with the SAPIEN 3 valve who they determine to have a predicted risk of surgical mortality of greater than or equal to 3 percent at 30 days, based on the Society of Thoracic Surgeons (STS) risk score and other clinical co-morbidities unmeasured by the STS risk calculator.
The SAPIEN 3 valve builds on Edwards' decades of experience in the development of tissue heart valves, and the proven benefits of the Edwards SAPIEN valves. The SAPIEN 3 valve was approved by the FDA in June 2015 for the treatment of patients with severe, symptomatic aortic stenosis who are at high-risk for open heart surgery.
About Edwards Lifesciences
Edwards Lifesciences, based in Irvine, Calif., is the global leader in patient-focused medical innovations for structural heart disease, as well as critical care and surgical monitoring. Driven by a passion to help patients, the company collaborates with the world's leading clinicians and researchers to address unmet healthcare needs, working to improve patient outcomes and enhance lives. For more information, visit www.Edwards.com and follow us on Twitter @EdwardsLifesci.
This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements include, but are not limited to, statements by Dr. Thourani and Mr. Wood and statements regarding expected product benefits and procedural outcomes. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict. Our forward-looking statements speak only as of the date on which they are made and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement.
Forward-looking statements involve risks and uncertainties that could cause the roll-out and benefits of the technology to differ materially from those expressed or implied by the forward-looking statements based on a number of factors including but not limited to unexpected outcomes after more expanded clinical experience, unexpected changes or delays related to product supply, potentials for unexpected regulatory or quality developments, competitive dynamics, litigation and customer acceptance. These factors are detailed in the company's filings with the Securities and Exchange Commission including its Annual Report on Form 10-K for the year ended December 31, 2015.
Edwards, Edwards Lifesciences, the stylized E logo, Edwards SAPIEN, Edwards SAPIEN 3, SAPIEN, SAPIEN 3, PARTNER and PARTNER II are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.