|
Biosensors International Announces Japanese PMDA
Approval for BioFreedom™ Ultra and US FDA Approval
for BioFreedom™ Biolimus A9™ Coated Polymer
Free Coronary Stent Systems
|
 |
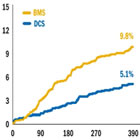
LEADERS FREE: BioFreedom DCS = 50% Reduction in TLR Over Bare Metal Stents at 390 Days |
April 21, 2022 -- Switzerland, Singapore -- Biosensors International Group, Ltd. ("Biosensors" or the "Company"), a developer, manufacturer and
supplier of innovative medical devices, is pleased to announce both Japanese PMDA Approval for
BioFreedom™ Ultra on 31st March 2022 and also US FDA approval for BioFreedom™ on 14th April 2022.
BioFreedom™ is Biosensors’, novel polymer and carrier-free DCS with their proprietary limus drug Biolimus
A9™(BA9™). BA9™ is Biosensors’ proprietary highly lipophilic anti-restenotic drug, developed specifically
for use in coronary vascular applications.
BioFreedom™ gives physicians the opportunity to reduce DAPT to one month in patients post-PCI who are
at High Bleeding Risk (HBR).
Biosensors Chief Executive Officer Yu Suhua said, “We are excited to launch these coronary stent systems
and broaden their availability in both Japan and the USA. The proven benefits of this Biolimus A9™ Coated
Stent System will help interventional cardiologists to further improve the clinical outcomes for many patients
requiring a stent, particularly the High Bleeding Risk patients”.
“Biosensors has been at the forefront of clinical research for the treatment of High Bleeding Risk Patients
undergoing PCI since the LEADERS FREE study was presented at TCT and published in the NEJM in
2015. It is great to finally be able to bring these technologies to Japan & the United States, “ commented
Biosensors Chief Medical Officer, Prof Keith Oldroyd.
The BioFreedom™ stent systems optimize the PCI procedure for High Bleeding Risk (HBR) patients by
simplifying stent choice pre-procedure. In the LEADERS FREE trial program, >2,500 HBR1 patients have
been successfully studied and treated with BioFreedom™ and only one month DAPT post procedure
followed by single antiplatelet therapy.
The LEADERS FREE II2 trial enrolled 1203 HBR patients at 66 sites across the USA and Europe, using the
same inclusion criteria as the LEADERS FREE3 randomized trial. LEADERS FREE II2 is a single arm trial
with all patients being treated using the BioFreedom™ DCS, with the BMS arm of LEADERS FREE used
as the control arm. The primary safety endpoint of the trial was a composite of cardiac death and myocardial
infarction, the primary efficacy endpoint was clinically driven target lesion revascularization. BioFreedom™
was both significantly safer (9.3% versus 12.4%; HR, 0.72 [95% CI, 0.55–0.94]; P=0.0150 for superiority)
and significantly more efficacious (7.2% versus 9.2%; HR, 0.72 [95% CI, 0.52–0.98]; P=0.0338 for
superiority) than the BMS at 1 year, in this previously understudied North American high bleeding risk
patient population.
BioFreedom is our first stent product to be approved in all the key markets globally, as well as many other
country specific registrations across the world.
About Biosensors International Group
Biosensors International has over 30 years in designing, manufacturing and marketing innovative medical
devices that improve patients' lives, including cardiovascular devices for Percutaneous Coronary
Intervention and structural heart devices for Transcatheter Aortic Valve Replacement (TAVR). The
company has worldwide operations through a combination of direct sales teams and distribution
networks. Biosensors has manufacturing facilities in Germany, Singapore and China. Its products are sold
in over 90 countries and regions. It is one of the world’s top four companies engaged in the research and
development, manufacturing and sales of stents and is a subsidiary of Blue Sail Medical.
For more information about Biosensors, please visit www.biosensors.com
Forward-Looking Statements
Certain statements herein include forward-looking statements which generally can be identified by the use
of forward-looking terminology, such as “may,” “will,” “expect,” “intend,” “estimate,” “anticipate,” “believe,”
“project” or “continue” or the negative thereof or other similar words. All forward looking statements involve
risks and uncertainties, including, but not limited to, customer acceptance and market share gains,
competition from companies that have greater financial resources; introduction of new products into the
marketplace by competitors; successful product development; dependence on significant customers; the
ability to recruit and retain quality employees as Bluesail Medical and Biosensors grow; and economic and
political conditions globally. Actual results may differ materially from those discussed in, or implied by, the
forward-looking statements. The forward-looking statements speak only as of the date of this release and
Bluesail Medical or Biosensors assumes no duty to update them to reflect new, changing or unanticipated
events or circumstances.
1. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. Urban p. et al. N Engl J Med. 2015;373(21):2038-47. S.Saito.
LEADERS FREE Japan study (single BioFreedom DCS arm with 1-month DAPT, compared to BMS arm of LEADERS FREE). ePoster
EuroPCR 2017. Global Approach to High Bleeding Risk Patients With Polymer-Free Drug-Coated Coronary Stents: The LF II Study. M.W.
Krucoff. Circ Cardiovasc Interv. 2020 Apr;13
2. Global Approach to High Bleeding Risk Patients With Polymer-Free Drug-Coated Coronary Stents: The LF II Study. M.W. Krucoff. Circ
Cardiovasc Interv. 2020 Apr;13
3. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. Urban p. et al. N Engl J Med. 2015;373(21):2038-47
4. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. Garot P et al. JACC VOL.6 9, NO.2 , 2017
5. Naber C. Biolimus-A9 polymer-free coated stent in high bleeding risk patients with acute coronary syndrome: a Leaders Free ACS sub-study
et al. Eur Heart J 2016;38:961-969
Source: Biosensors International Group, Ltd., April 21, 2022 |

