
|
|
|
 |
 |
 |
The following article provides
an overview of drug-eluting stents (a.k.a "drug
coated stents" or "medicated stents" or
"DES") which gives a basic understanding of the history
and
mechanisms of these devices
For more in-depth information and late-breaking
articles about drug-eluting stents, visit our Drug-Eluting
Stent Center.
|
|
|
|
 typical coronary stent
typical coronary stent
from the early 90's |
|
A
Brief History of Stenting
The concept of the stent grew
directly out of interventional cardiologists' experience
with angioplasty balloons in the first decade of use
(1977-87). Sometimes the wall of the coronary artery
became weakened after balloon dilatation. Although
the artery would be opened successfully using a balloon,
in a small percentage of cases, the artery would collapse
after the balloon was deflated -- sometimes this might
not happen until the patient had been moved to the
recovery room. Since there was no interventional "fix" available,
the only option for this patient was emergency bypass
graft surgery to repair the problem. |
|
The Dilemma of Restenosis
A second problem soon became
evident as well. 30-40% of all coronary
arteries began to close up again after balloon angioplasty.
By the mid-80's various radiologists and cardiologists
were working on solutions to these problems, designing
new devices in hopes they would provide more safety
and durability to the procedures. Lasers, tiny "shavers",
rotational "polishers" -- many tools were
miniaturized to be delivered via catheter.
|
|
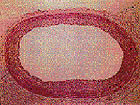 cross section of
cross section of
restenosed artery |
|

Close-up of early
Palmaz-Schatz stent
|
|
The First
Stents
One such device was the stent
-- a metal tube or "scaffold" that was
inserted after balloon angioplasty. The stent itself
was mounted on a balloon and could be opened once
inside the coronary artery. Julio Palmaz and Richard
Schatz were working on such a stent in the United
States; others in Europe were developing their own
designs. In 1986, working in Toulouse, France, Jacques
Puel and Ulrich Sigwart inserted the first stent
into a human coronary artery. In 1994 the first Palmaz-Schatz
stent was approved for use in the United States.
Over the next decade, several generations of bare
metal stents were developed, with each succeeding
one being more flexible and easier to deliver to
the narrowing.
|
|
A Persistent
Problem
But while stents virtually
eliminated many of the complications of abrupt artery
closure, restenosis persisted. Although the rates
were somewhat lower, bare metal stents still experienced
reblocking (typically at six-months) in about 20-25%
of cases, necessitating a repeat procedure. The interventional
cardiology community also learned that restenosis,
rather than being a recurrence of coronary artery
disease, actually was the body's response to what
Andreas Gruentzig called the "controlled injury" of
angioplasty and was characterized by growth of smooth
muscle cells -- roughly analogous to a scar forming
over an injury.
|
|
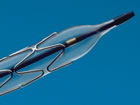 Close-up of stent
mounted on a balloon, circa 1995
Close-up of stent
mounted on a balloon, circa 1995 |
 A drug-eluting stent,
A drug-eluting stent,
circa 2002 |
|
Development
of Coated and Drug-Eluting Stents
More and more, the solution
moved away from the purely mechanical devices of
the 90's and toward pharmacologic advances that
were being made. If interventional medicine, using
the body's circulatory system as a "highway" to
deliver therapy, worked with devices, it could
also work with medicines. Physicians and companies
began testing a variety of drugs that were known
to interrupt the biological processes that caused
restenosis. Stents were coated with these drugs,
sometimes imbedded in a thin polymer for time-release,
and clinical trials were begun.
|
Drug-Eluting Stent
Basics
Sometimes referred to as
a “coated” or “medicated” stent,
a drug-eluting stent is a normal metal stent that
has been coated with a pharmacologic agent (drug)
that is known to interfere with the process of
restenosis (reblocking). Restenosis has a number
of causes; it is a very complex process and the
solution to its prevention is equally complex.
However, in the data gathered so far, the drug-eluting
stent has been extremely successful in reducing
restenosis from the 20-25% range to single digits. There
are three major components to a drug-eluting stent:
-
Type
of stent that carries the drug coating
-
Method
by which the drug is delivered (eluted)
by the coating to the arterial wall (polymeric
or other)
-
The
drug itself – how does it act in
the body to prevent restenosis?
|
|
|
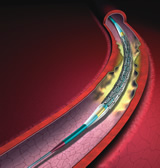 Artist's rendition
of coated stent on balloon in artery
Artist's rendition
of coated stent on balloon in artery |
 Interventional procedure
being performed in the catheterization lab
Interventional procedure
being performed in the catheterization lab |
|
In
addition, there are several decisions made by the interventional
cardiologist that result in a successful stent placement,
whether of the drug-eluting or bare metal variety:
- Correct sizing of
the stent length to match the length
of the lesion, or blocked area
- Correct sizing of
the stent diameter to match the thickness
of the healthy part of the artery
- Sufficient deployment
of the stent, making sure that the stent,
once placed at the optimum site in the
blocked artery, is expanded fully to
the arterial wall – under-expansion
can result in small gaps between the
stent and arterial wall which can lead
to serious problems such as blood clots,
or Sub-Acute Thrombosis (SAT)
|
|
Usually
the sizing and the assessments of expansion are made
by viewing the real-time angiogram in the cath lab,
although some cardiologists also are using more detailed
information obtained through intravascular ultrasound
imaging.
Finally, in addition to aspirin, the patient must
take an anti-clotting or antiplatelet drug, such
as clopidogrel, prasugrel or ticlopidine (brand names
Plavix, Effient or Ticlid) for a year or more after
the
stenting, to
prevent the blood from reacting to the
new device
by thickening and clogging up the newly expanded
artery (thrombosis). Ideally a smooth, thin layer
of endothelial cells (the inner lining of the blood
vessel) grows over the stent during this period and
the device is incorporated into the artery, reducing
the tendency for clotting.
|
|
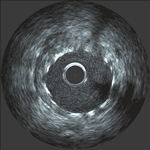 Intravascular ultrasound
image of stent in artery
Intravascular ultrasound
image of stent in artery |
|
|
|
"Stent
Wars"
The status and availability
of drug-eluting stents have been the subject
of many legal disputes and other issue, which
some
time
ago Angioplasty.Org labeled "Stent Wars".
These devices were initially adopted so quickly
that
they doubled
the world market for stents to $5 billion annually.
While usage of drug-eluting stents was reduced
immediately after concerns over stent thrombosis
first surfaced in 2006, with further studies showing
increased efficacy and the introduction of newer
second and
third
generation
drug-eluting stents, DES use is again increasing.
Currently in the U.S., the DES market is shared
by Abbott, Boston Scientific and Medtronic. Cordis/J&J,
manufacturer of the CYPHER, the first FDA-approved
DES, was unable produce a second generation
device and so announced in 2011 that it was ceasing
production of the CYPHER and dropping out of the
stent market entirely.
|
|
The newer drug-eluting stents have
thinner, more flexible struts, thinner and more biocompatible
polymers that elute the drug and, in most randomized
clinical trials and observational studies, all show
increased efficacy and safety: both the risks of stent
thrombosis and the
rate of restenosis have been reduced from the first
generation devices.
Taking a different approach,
both Abbott and Germany-based Biotronik are testing
a completely bioabsorable stent
which will
totally disappear after it has done its work. OrbusNeich
received CE mark approval in 2005 for its Genous stent
which, rather than using drugs to suppress excess tissue
growth, utilizes an bio-engineered coating to attract
a thin "all-natural" endothelial layer within
hours. A number of other companies, such as Biosensors,
Stentys, etc, are working on other drug/polymer/stent
combinations.
The major positive for drug-eluting
stents is that all the approved devices
have shown significant reduction of restenosis in clinical
trials
and in the "real world". DES have also shown
dramatic reduction in reinterventions in diabetics
as well --
this is
a population
that has been highly susceptible to restenosis in the
past.
Stent Thrombosis
There is some evidence
that drug-eluting stents may be susceptible to an
event known as "late
stent thrombosis", where the blood-clotting inside
the stent can occur one or more years post-stent, after
the recommended one-year of dual antiplatelet therapy
has ended. While this was seen infrequently
in the first-generation Taxus and
Cypher
stents, thrombosis is extremely dangerous, fatal in
over one third of cases. To prevent thrombosis, a full
course of post-stent antiplatelet
therapy is
very important and patients should not stop taking
aspirin, Plavix, Effient or Ticlid without consulting
their interventional cardiologist.
The issue of late stent thrombosis,
although discussed within the profession since drug-eluting
stents were
introduced, received widespread publicity at the September
2006 World Congress of Cardiology meeting in Barcelona
when three European studies pointed to higher rates
than
had previously been seen (see our feature, "Problems
Resurface with Drug-Eluting Stents" for detailed
coverage). Late stent thrombosis was one of the major
issues discussed at the 2006 TCT Meeting and
the FDA scheduled a public meeting in early December
2006
on the issue. The conclusion was that more information
was needed, especially about the use of devices in
off-label settings, but that when used as directed,
there did not seem to be greater risks of death or
heart attack with drug-eluting stents.
However, within a short time, newer
second-generation drug-eluting stents came to market
and studies have shown the risk of late stent thrombosis
to be even lower. In fact the Swedish Stent Registry
(SCAAR) which reported great concerns over stent thrombosis
in 2006, has now shown the exact opposite, with drug-eluting
stents showing far great safety and effectiveness than
bare metal stents.
Developments in this field can
be fast and furious. Keep abreast of the constantly
changing
status of clinical trial
results and controversies in our Drug-Eluting
Stent NewsCenter.
|
|
|
|
Outlook
for Patient Care
While there continue
to be questions about the use (or overuse) of
drug-eluting stents vs. medical therapy alone,
and which is the best stent,
the development of these sophisticated devices
and the new wave of treatment options are further
expanding the tools of cardiologists. The
durability of interventional procedures to treat
coronary
artery disease non-surgically
has increased exponentially with the introduction
of drug-eluting stents. The specter, possible complications
(and expense) of repeat procedures, will be vastly
reduced. And diabetics, a patient population that
previously has not seen great success with angioplasty
and stent procedures, can now be treated with more
confidence.
|
|
| |
|
|