![]()
<< To Homepage >>
<<Archives>>
February 2010 Archives:
February 19, 2010 -- 6:35pm EST Plavix After Stents: How Long? Sort of shocking. A major study was supposed to come out of those hearings, but the DAPT study just began recruiting last summer and won't be completed for four years. This massive study, sponsored by all the major stent makers, as well as the manufacturers of antiplatelet meds, will enroll 20,000 patients and test them at 12 and 30 months to determine the rates of MACCE (death, heart attack and stroke) stent thrombosis and major bleeding complications. It will be performed with all drug-eluting stent brands and will not compare one to another. What will this teach us? Dr. Topol has an opinion about the DAPT study:
Dr. Topol (who, by the way, was an invited panel member at those 2006 FDA hearings) is currently Director of the Scripps Translational Science Institute (STSI) where he is conducting research on the genomics of coronary artery disease. The bottom line is that all patients are not the same -- and they respond to antiplatelet therapy differently. So Dr. Topol believes that the "one-size-fits-all" concept of thrombosis prevention just doesn't apply. Another concept is that all drug-eluting stents aren't the same. Because of the metal structure, polymer coating or drug itself, each device has different characteristics and different healing properties. This was seen clearly in the ODESSA trial, where Dr. Giulio Guagliumi used OCT intravascular imaging to measure stent coverage at six months. He found significant incomplete coverage in the CYPHER and TAXUS stents, but complete healing in the ENDEAVOR. As a result of these findings and other clinical data, two trials, involving only Medtronic's ENDEAVOR stent, are currently starting up: SEASIDE, which Dr. Topol is involved in, will measure the outcomes of patients who receive and only get six months of DAPT; and OPTIMIZE, being conducted in Brazil by Dr. Fausto Feres, which is stopping DAPT at three months. Rather than testing if DAPT is more effective at longer durations, such as 12 and 30 months, these studies are testing to see if it is just as effective at shorter periods, when used with a DES that has a greater healing profile, like the ENDEAVOR. The advantages of a shorter DAPT duration are several:
The short back story here is that when drug-eluting stents first came on the market in 2003-2004, the FDA recommended six months of DAPT to keep the blood from clotting in and around the stent (a.k.a. stent thrombosis). Within a couple of years, reports surfaced about a small number of patients who suffered late stent thrombosis (six months or more after stenting). A flurry of concern arose and the 2006 FDA stent safety hearings resulted in recommendations to extend DAPT to 12 months or more -- the current guidelines. But, as Dr. David Kandzari, co-principal investigator for the SEASIDE trial told Angioplasty.Org:
Dr. Kandzari explores this issue in detail in his Viewpoint article in December's JACC: Interventions, "Identifying the 'Optimal' Duration of Dual Antiplatelet Therapy After Drug-Eluting Stent Revascularization" For more information, read my interview with Dr. Topol and keep up with the latest news in our StentCenter. « permalink » « send comment » « back to top »
February 16, 2010 -- 10:55pm EST Stents Downgraded by Wall Street Journal:
If Only It Were That Simple The main thesis of Winstein's piece is that the use of stents, after an initial drop of 13% right after COURAGE was published, is now back to pre-COURAGE levels. With the subtitle, "Why Health Policies Can Fail to Keep Up With Key Medical Findings", the WSJ article questions this rebound in stent use, which it characterizes as a "lucrative treatment" that can be "ineffective" and "unnecessary", and discusses the resistance to the COURAGE results from both the interventional cardiology community and the medical device manufacturers. (Gee...and I always thought the WSJ was a "friend" to business and industry.) The article also discusses how reimbursement policies currently favor stenting over medical therapy. WSJ Simplifies Study Results by Ignoring
Key Issues
The words that jump out at me are "simple" and "straightforward" -- because the diagnosis and treatment of coronary artery disease are neither. It may be comforting to view medicine through the eyes of an engineer or programmer, where you define a problem, create a fix, test it and implement it. But figuring out how to diagnose and treat a specific patient involves juggling multiple moving targets while keeping on top of the latest research, findings and available tools: it's what good doctors do. Also Winstein's choice of the phrase "no additional benefit" is not accurate. The COURAGE study concluded that the addition of stenting "did not reduce the risk of death, myocardial infarction, or other major cardiovascular events." In fact, the Quality of Life portion of COURAGE showed that patients who received stents felt better (less pain) than the patients on OMT. That would definitely be a "benefit". So here are a few facts and findings that may mess up this "simple concept" and show it to be a bit less than straightforward: Comparison About More Than Just Drugs
vs. Stents 1/3rd of Patients Given "Drugs
Only" Switched to Stents Study included only Bare Metal Stents Very Limited Patient Selection -- 90%
of Candidates Rejected Pre-Testing No Simple Solution Either Guidelines Plus Individualized Medicine
-- More Effective than One Size Fits All! What we really need is better patient and professional education, and a system that supports patient self-care, which might increase implementation of these guidelines. But making that happen is a hard sell -- probably because it doesn't immediately make or save anyone money (take it from those of us in the education business!). The take-away from COURAGE is that, in this very specific low risk patient population, stenting can be safely deferred, to see if OMT can provide a less invasive, less costly benefit. The downside for patients, however, is if insurers take some of these concepts and use them to deny care. In an interesting experiment, Blue Cross/Blue Shield in some parts of New York is requiring "stable patients" to do 12 weeks of medical therapy before they will cover a stent procedure. I only wonder if the type of COURAGE-level support will be there for them. I also wonder how the type of patient that would be in the COURAGE cross-over-to-stenting group will fare. Will they have to suffer angina for weeks before their procedure can be covered by insurance? Finally, there is a strong movement in medicine today towards individualized treatment. As more is learned of genetic markers, cytochromes, etc. it may be that "one size fits all" treatment will disappear. This concept has been discussed at length by many cardiologists, most directly by Dr. Eric Topol regarding optimal duration of antiplatelet therapy. (Read his exclusive interview on Angioplasty.Org) So the fear of using results from a study of a very particular hand-picked although large patient population to mandate (and possibly limit) care for a broad population of heart patients, is a bit disconcerting. Remember too that in all of this, we are only discussing those patients with "chronic stable angina", a condition which itself many have trouble defining. Not in dispute are patients with more serious coronary artery disease, especially those in the midst of a heart attack. They are, without any question, best served by reopening the arteries, whether via bypass surgery or angioplasty. « permalink » « send comment » « back to top »
February 12, 2010 -- 4:30pm EST Clinton and Eisenhower: Presidents, Hearts,
Stents and 55 Years Whatever your take on comparative effectiveness research, whether too many stents are used, etc., you have to admit it's pretty amazing. The advances made in diagnosis, bypass surgery and interventional catheter-based techniques have revolutionized the treatment of coronary artery disease. Especially when you look back at how this illness used to be treated (or not). The take-away from Bill Clinton's episode is that even though his quadruple bypass surgery was successful, the natural history of coronary artery disease is that it will progress. Surgery, angioplasty, stents, medicine -- none of these are cures, but they are interventions in this progression. Along with the medications, exercise, diet and smoking cessation, the natural history can be slowed down. Clinton, having experienced this pain previously, knew it was significant and did precisely the right thing: he saw his cardiologist. « permalink » « send comment » « back to top »
February 11, 2010 -- 8:00pm EST IVUS vs. FFR -- Boston Style So what's wrong with this picture? Well...FFR measures intracoronary blood pressure across a blockage. A blockage may look significant on an angiogram, but 1/3 of the time (according to the FAME study) it isn't "flow-limiting"-- and probably doesn't need to be opened up and stented. Working this way is called FFR-guided PCI (i.e. angioplasty and stenting). It improves outcomes and saves money. In fact the recently issued AHA/ACC/SCAI Guidelines Update just raised the level of evidence for use of FFR to "A", stating that FFR "can be useful to determine whether PCI of a specific coronary lesion is warranted." IVUS (IntraVascular UltraSound) is an imaging modality and it doesn't measure blood pressures. It allows the cardiologist to see (and measure quite accurately) the diameter of the artery, the size of the blockage, the length of the diseased portion. In newer sophisticated systems, something called Virtual Histology™ can even differentiate the type of plaque, including so-called "vulnerable plaque" that may be more prone to causing a heart attack. IVUS is a great tool, kind of like having a ruler inside the artery, but the revelations of the FAME study could not have been made using IVUS. In fact the two tools can work very well together: FFR-guided PCI refines the decision-making process of whether or not to stent; IVUS refines the mechanical stenting process of placing and inflating the device optimally. Ideally, both can be used to improve the outcomes of PCI procedures. It's not either/or: IVUS helps you do a better job when you stent people; FFR helps you avoid stenting people unnecessarily. So why is Boston Scientific demeaning the value of Fractional Flow Reserve? Possibly because there are only two manufacturers of this technology, and they're not one of them. St. Jude is one, as a result of their acquisition of Swedish firm Radi; Volcano is the other. Volcano is also Boston Scientific's only competitor in the IVUS field -- and they offer a system that integrates both FFR and IVUS. The ad suddenly made sense. As for my search for information on FFR, I ran into a familiar situation. The first two hits were articles I had already written on the subject! « permalink » « send comment » « back to top »
February 9, 2010 -- 11:50pm EST Volcano Has AAA Week: Accused, Approved
and Acquired But today, Volcano made two more positive announcements. The FDA has approved Volcano's latest IVUS catheter, the Eagle Eye® Platinum. It represents the latest refinement in intravascular ultrasound imaging (see picture above) -- a modality which has been shown to improve stent placement and sizing. And also today, Volcano announced that it has acquired the Xtract™ Thrombus Aspiration Catheter from Lumen Biomedical. This device is sort of a vacuum cleaner, used before angioplasty and stenting during a heart attack to remove the blood clot that stops the blood flow. Thrombus aspiration has been shown to significantly improve patient outcomes. Looking at these new developments, and given that Volcano's pipeline includes forward-looking IVUS and other future therapeutic modalities, it's clear that the company is compiling an armamentarium of both imaging and treatment devices, all for delivery with the same console in the cath lab: a variation on "see one, do one". (Volcano's stock closed at $19.84 today, up 33% from a year ago.) « permalink » « send comment » « back to top »
February 7, 2010 -- 11:10pm EST Herbal Supplements vs. Heart Drugs: Controversy
Re-ignited
The JACC article details the significant amounts of money ($34.4 billion) expended annually in the U.S. on Complementary and Alternative Medicine (CAM). The authors calculate over $5 billion is spent out-of-pocket on herbal supplements alone. Yes, that's $5 billion -- the total worldwide market for drug-eluting stents -- perhaps executives at Medtronic or Boston Scientific might be thinking, "maybe we're in the wrong business". In any case, the JACC paper details a number of herbs, natural substances and preparations that have an impact on the cardiovascular system -- from helping lower high blood pressure by dilating arteries, to increased antiplatelet effects to keep blood from clotting. These all sound like useful and good qualities. But, as the authors point out, problems may occur, as in "too much of a good thing". The CAM forces are unhappy with what they claim is the implication that herbal and natural supplements are unsafe. But I think this misses the major point of the paper. Quite the opposite, the paper details the ways in which herbal supplements are powerful medicines. If, for example, you have had a stent implanted and are on dual antiplatelet therapy (aspirin and clopidogrel) to keep from developing dangerous blood clots in the stent, you may think it would be a good idea to also take Ginkgo for "cardiovascular health" to keep the blood even more slippery. Yet Ginkgo intensifies the antiplatelet drugs to the point where bleeding complications may arise and may cause internal hematoma or hemorrhage. So it's not that ginkgo is unsafe -- it's that it has a very definite effect. And so with other supplements that affect the cardiovascular system. Some covered in the JACC article are: St. John's wort, motherwort, ginseng, garlic, grapefruit juice, hawthorn, saw palmetto, danshen, echinacea, tetrandrine, aconite, yohimbine, gynura, licorice, and black cohosh. The important point of this paper is that patients and physicians should be talking to each other about alternative supplements, and making sure they are taken into account when drawing up a medical management plan -- to ensure there are no interactions or unwanted enhanced effects. To rebut the implication that physicians currently do not query patients about herbal supplements, CRN has published the results of a survey, showing that, in fact, patients and cardiologists DO discuss them to a high degree. I wonder if that is really the norm and would welcome comments. « permalink » « send comment » « back to top »
February 5, 2010 -- 8:40pm EST Patent Spending But no such hyperbole for this week's stent news. Instead, on Monday, Boston Scientific announced (rather quietly) that, rather than go to jury trial later this month on patent disputes with Johnson & Johnson / Cordis that date back to 2003, they've decided to just settle everything -- for a mere $1.725 billion! Consider that this price tag is the same quantum as Toyota's hit, and consider that, as the Wall Street Journal points out, not only is this amount almost double J&J's total 2009 sales of drug-eluting stents, it may eclipse Boston's as well. So one might wonder why, other than a few scattered articles in the business press, there's been so little publicity regarding this major concession. After all, these disputes have been in the courts for years, and many millions already have been spent on lawyers' fees, etc. One can only assume that Boston Scientific foresaw a worse outcome from a jury trial and so, decided on the option of settlement. Perhaps the company saw the futility of trying to challenge the original stent patents of Palmaz and Gray, patents that time and again have been upheld. But a worse outcome than $1.725 billion?
Ray Elliott, Boston's new CEO, stated that the company settled to "mitigate risk" and to resolve "major litigation without exposing Boston Scientific to the uncertainties of a jury trial and a potential damages award that was impossible to predict." But the price tag is very high and a stock analyst, quoted in Tuesday's WSJ, opined, "We think most investors will be surprised and concerned," by the new deal. As for Dr. Palmaz, he will no doubt be relieved at being able to spend February and March elsewhere. |
 I
recently
I
recently  A
broad-based critique, claiming overuse of heart stents and angioplasty,
was published as a major feature in Thursday's Wall Street
Journal. Clocking in at just under 2,000 words, Keith J.
Winstein's article, "
A
broad-based critique, claiming overuse of heart stents and angioplasty,
was published as a major feature in Thursday's Wall Street
Journal. Clocking in at just under 2,000 words, Keith J.
Winstein's article, " It's
a holiday concurrence: Valentine's Day and President's Day and
American Heart Month -- and former President Bill Clinton who
got his heart fixed six years ago and just
It's
a holiday concurrence: Valentine's Day and President's Day and
American Heart Month -- and former President Bill Clinton who
got his heart fixed six years ago and just  Dr.
William W. O'Neill, Professor & Executive Dean for Clinical Affairs,
University of Miami, Division of Cardiology has a favorite lecture
that he gives on this topic. He goes back to 1955 and describes how
then President Eisenhower's chest pains were first diagnosed as gastric
upset and finally almost a day later an EKG showed he was in the
midst of a massive heart attack. But there was nothing any doctor
could do then, except give Ike morphine for the pain. The heart attack
had to play itself out, Ike's heart muscle was damaged and he was
in the hospital for 7 weeks. He didn't return to work for 3 months.
(for you young folk, our Vice President at the time was Richard Nixon!).
Ike did continue as President and, in fact, was re-elected to a second
term (after all, he was THE hero of WWII). But without question,
his ability to lead a fully active life was significantly compromised
and ultimately, in 1967, he succumbed to heart disease. (A print
version of Dr. O'Neill's lecture can be found
Dr.
William W. O'Neill, Professor & Executive Dean for Clinical Affairs,
University of Miami, Division of Cardiology has a favorite lecture
that he gives on this topic. He goes back to 1955 and describes how
then President Eisenhower's chest pains were first diagnosed as gastric
upset and finally almost a day later an EKG showed he was in the
midst of a massive heart attack. But there was nothing any doctor
could do then, except give Ike morphine for the pain. The heart attack
had to play itself out, Ike's heart muscle was damaged and he was
in the hospital for 7 weeks. He didn't return to work for 3 months.
(for you young folk, our Vice President at the time was Richard Nixon!).
Ike did continue as President and, in fact, was re-elected to a second
term (after all, he was THE hero of WWII). But without question,
his ability to lead a fully active life was significantly compromised
and ultimately, in 1967, he succumbed to heart disease. (A print
version of Dr. O'Neill's lecture can be found  Earlier
today I was doing research for an article about stents for
our site,
Earlier
today I was doing research for an article about stents for
our site, 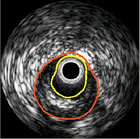 Volcano
Corporation (Nasdaq: VOLC) has had a pretty interesting week.
Last Thursday was the low point: a 13 person jury in Massachusetts
Superior Court found Volcano and its subsidiary Axsun
Volcano
Corporation (Nasdaq: VOLC) has had a pretty interesting week.
Last Thursday was the low point: a 13 person jury in Massachusetts
Superior Court found Volcano and its subsidiary Axsun  An
article, that should be of special concern to stent and angioplasty
patients, appears in the current Journal of the American
College of Cardiology (JACC). This "state-of-the-art
paper" from the Mayo Clinic, titled "
An
article, that should be of special concern to stent and angioplasty
patients, appears in the current Journal of the American
College of Cardiology (JACC). This "state-of-the-art
paper" from the Mayo Clinic, titled " Julio
Palmaz, inventor of the original stent design, told me recently that,
if you wanted to be an inventor, you'd better be prepared to spend
a lot of time in court. Over the past two decades, Palmaz spent years
sitting in courtrooms all over the world. In fact, he sold his patents
to J&J in 1998 partly to eliminate the suspicion of personal
gain when he testified on behalf of his invention. As he told the New
York Times in
Julio
Palmaz, inventor of the original stent design, told me recently that,
if you wanted to be an inventor, you'd better be prepared to spend
a lot of time in court. Over the past two decades, Palmaz spent years
sitting in courtrooms all over the world. In fact, he sold his patents
to J&J in 1998 partly to eliminate the suspicion of personal
gain when he testified on behalf of his invention. As he told the New
York Times in