|
October 22, 2008 / Washington, DC -- A new imaging technology, Optical Coherence Tomography (OCT) popped up in a number of presentations and evening symposia at this year's Transcatheter Cardiovascular Therapeutics meeting (TCT 2008). OCT is very new: one of the two currently available systems was first used in humans only five months ago.
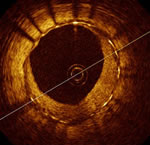
But OCT allows viewing the interior surfaces of the artery and stent in an awake patient. The imaging is done in the cath lab, over the same type of wire as balloons, stents, IVUS, etc. devices are delivered. The assessment of strut coverage in stents was the goal of the ODESSA Trial (Optical coherence tomography for DES SAfety). Presented last Tuesday by Dr. Giulio Guagliumi of Ospedali Riuniti di Bergamo, Italy, the goal of this trial was to determine whether OCT could accurately measure strut coverage and stent malapposition in long lesions (greater than 20mm) that required overlapping stents. Overlapping stents are associated with an increased incidence of uncovered struts. Although the number of patients (77) and stents (189) was small, the actual number of struts analyzed was 53,047. The stents were further divided in to four groups: sirolimus-eluting (SES) or CYPHER stents, paclitaxel-eluting (PES) or TAXUS stents, zotarolimus-eluting (ZES) or ENDEAVOR stents, and bare-metal stents (BMS). Also measured was the amount of neo-intimal hyperplasia (NIH): the amount of tissue regrowth inside the stent. Too much NIH and restenosis occurs: the stent closes up. Too little NIH may result in delayed healing and increased stent thrombosis, especially late stent thrombosis which can occur a year or more after implantation.
Reviewing the ODESSA Trial for the American College of Cardiology's Cardiosource, Dr. Dharam J Kumbhani of the Cleveland Clinic concluded: "While the OCT findings are unique and interesting, the clinical significance of these findings is unclear. Moreover, even in this small study, the investigators had to analyze more than 50,000 struts. Hence, until this methodology is standardized, automated, and validated, its applicability in routine practice is limited." Angioplasty.Org expects to see the use of OCT increase in future clinical trials and may, at some future time, be useful in determining when it is safe for DES patients to stop dual antiplatelet therapy.
Reported by Burt Cohen, October 22, 2008 (updated) |
|||||||||||||||||||||||||||||||||||||