|
Volcano Corporation Announces Receipt of CE Mark for Its OCT Imaging System and Catheter
|
 |
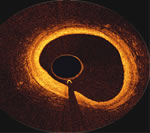
OCT Image
inside right coronary artery |
|
January 5, 2010 -- San
Diego -- Volcano Corporation (Nasdaq: VOLC), a leader in the development,
manufacture and sales of products for the diagnosis and treatment
of coronary and peripheral artery disease, announced today receipt
of CE mark for their OCT (Optical Coherence Tomography) imaging
system and catheter.
Volcano's OCT product line is expected to
complement its existing line of IVUS imaging catheters and
pressure guide wires used for coronary imaging and lesion assessment. |
"This is an important milestone in our development of OCT and highlights the progress of our OCT program overall," noted Vince Burgess, President of Advanced Imaging Systems at Volcano Corp. "Volcano
acquired its initial OCT technology through the acquisition of
CardioSpectra, Inc. in December 2007. Since that time, the Volcano
OCT system has generated positive results in numerous clinical
studies in Europe and South America. Building upon the CE mark
and pending IDE approval, Volcano plans to use this system in the
US and South America as part of a US regulatory trial during 2010.
We currently expect commercial release of our first OCT system
in Europe in early 2011."
Scott Huennekens, President and
Chief Executive Officer of Volcano, commented, "Volcano is committed
to expanding our technology leadership in intravascular imaging,
both to complement everyday PCI (percutaneous coronary intervention)
technique, and to further the understanding of atherosclerotic
disease progression. We are working on products that incorporate
OCT functionality into our s5i integrated platform, as well as
stand-alone OCT systems. Our emphasis continues to be focused around
providing a variety of therapy enabling modalities so that the
physician can select which technology is most appropriate for each
individual patient."
| Marc D. Feldman, M.D., Professor
of Medicine and Director of the Cath lab at UTHSC San Antonio,
Texas commented, "This is an important milestone in the translation
of OCT imaging to everyday patient care. The Volcano OCT system
is designed to eventually allow the interrogation of an entire
coronary artery without concerns regarding patient safety due
to larger volume injections or balloon occlusion. The ability
to safely image a coronary artery can be utilized for image guided
PCI with fast, easy lumen and stent assessments. OCT can also
visualize intraluminal thrombus and dissections, as well as assist
in the evaluation of newer generations of drug eluting stents
and in the assessment of intimal coverage in previously paced
stents. It can also allow physicians to evaluate lesion cap thickness
down into the 15 micron range. As cardiologists, we spend billions
of dollars every year on coronary stents which have been proven
to benefit patients. Technologies like OCT should help us better
select the most significant blockages to treat, and help us ensure
these stents are placed properly." |
|

Marc D. Feldman,
MD, FACC,
UTHSC San Antonio, Texas |
"This is another step in what Volcano sees as the evolution of OCT," added Mr. Burgess. "We
will continue to make improvements to our OCT products and with
the addition of the HDSS (High Definition Swept Source) light source
developed by our team at Axsun Technologies (a wholly-owned subsidiary
of Volcano Corporation), we expect our next generation system to
be able to scan the proximal 2/3 of the coronary artery in as little
as one second. For this next generation OCT system, our team is
focused on enabling our Low Volume OCT (LVOCT(TM)) imaging system.
Developing the LVOCT(TM) imaging system will be the key to allowing
interventional cardiologists to acquire OCT images during a routine
coronary angiographic injection. This extremely low flush volume
is expected to allow for optimal imaging while maximizing patient
safety, clinical utility and ease-of-use for the physician and
staff."
About Volcano Corporation
Volcano Corporation (Nasdaq: VOLC) offers a broad suite of devices designed to facilitate endovascular procedures, enhance the diagnosis of vascular and structural heart disease and guide optimal therapies. The company's intravascular ultrasound (IVUS) product line includes ultrasound consoles that can be integrated directly into virtually any modern cath lab. Volcano IVUS offers unique features, including both single-use phased array and rotational IVUS imaging catheters, and advanced functionality options, such as VH(R) IVUS tissue characterization and ChromaFlo(R). Volcano also provides functional measurement (FM) consoles and single-use pressure and flow guide wires, and is developing a line of ultra-high resolution Optical Coherence Tomography (OCT) and Forward Looking IVUS systems and catheters. Currently, more than 4,700 Volcano
IVUS and FM systems are installed worldwide, and more than half of Volcano's revenues are derived from outside the United States. Through its wholly-owned subsidiary, Axsun Technologies, Volcano also develops and manufactures optical monitors, lasers and optical engines used in telecommunications, spectroscopy and other industrial applications. These products are sold to a variety of customers, including Nokia, Siemens, Ericsson, Alcatel-Lucent and HuaWei Technologies. For more information, visit the company's website at http://www.volcanocorp.com.
Forward-Looking Statements
This press release contains forward-looking
statements within the meaning of the U.S. Private Securities Litigation Reform
Act of 1995. Any statements in this release regarding Volcano's business that
are not historical facts may be considered "forward-looking statements," including
statements regarding the potential benefits of the systems, products and procedures
described above, results and implications of the data from clinical studies,
expected regulatory trials and commercial release and market adoption of the
company's technology, and the impact of clinical and other technical data.
Forward-looking statements are based on management's current preliminary expectations
and are subject to risks and uncertainties which may cause Volcano's results
to differ materially and adversely from the statements contained herein. Some
of the potential risks and uncertainties that could cause actual results to
differ from the results predicted are detailed in the company's annual report
on Form 10-K, quarterly reports on Form 10-Q and other filings made with the
Securities and Exchange Commission. Undue reliance should not be placed on
forward-looking statements which speak only as of the date they are made. Volcano
undertakes no obligation to update any forward-looking statements to reflect
new information, events or circumstances after the date they are made, or to
reflect the occurrence of unanticipated events.
Source: Volcano
Corporation
|



