Importantly, although high blood pressure puts one in three adults worldwide at risk of heart attack, stroke and kidney failure, the renal denervation studies that have been conducted to date have looked only at reducing blood pressure rather than the overall impact on major cardiac events. (St. Jude Medical announced last week just such a study: the EnligHTN II trial.)
St. Jude's (NYSE:STJ) announcement today of the EnligHTNment trial is the first that will look at "hard end points" such as heart attack, stroke and mortality. A spokesperson from St. Jude told Angioplasty.Org that specifics about the trial, such as target enrollment, number of centers, inclusion/exclusion criteria and follow-up period will be forthcoming.
St. Jude's EnligHTN™ Renal Denervation System is one of several such devices currently being studied. Others include Medtronic's Symplicity™ which has over five years of clinical experience and is currently marketed in parts of Europe, Asia, Africa, Australia and the Americas, as well as Vessix's V2™ Renal Denervation System, Covidien's OneShot™ System, and Recor's Paradise™ System. All have achieved the CE mark, but to date none have FDA approval for use in the United States.
A press release from St. Jude Medical, Inc. announcing the EnligHTNment trial follows:
St. Jude Medical Initiates Landmark Study of Renal Denervation for Reduction of Heart Attack, Stroke and Death
EnligHTNment trial will evaluate whether patients with hypertension that are treated with renal denervation and medication experience additional benefits beyond a reduction in blood pressure
February 15, 2013 -- St. Paul, Minnesota -- St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, today announced plans for a new landmark study that will evaluate whether renal denervation and medication can provide health benefits to patients beyond lowering high blood pressure. The EnligHTNment trial is the first large-scale study that will examine the long-term effects of renal denervation in patients who have uncontrolled hypertension to see if renal denervation also reduces the risk of major cardiovascular events such as heart attack, stroke and death.
Uncontrolled hypertension occurs when blood pressure in the arteries remains elevated, requiring the heart to work harder than normal to circulate blood throughout the body. This condition puts one in three adults worldwide at risk of heart attack, stroke and kidney failure according to the World Health Organization (WHO). Additionally, the WHO estimates that 7.5 million deaths each year, or 13 percent of all deaths can be attributed to raised blood pressure. This includes 51 percent of deaths due to stroke and 45 percent of deaths due to coronary heart disease.
"To date, the renal denervation studies that have been conducted only looked at reducing blood pressure in patients with uncontrolled or resistant hypertension," said Professor Michael Böhm, director and chief of internal medicine and cardiology at the University of Saarland in Homburg/Saar, Germany, a principal investigator for the trial. "What we need to know is if this minimally invasive approach for treating hypertension also correlates to a reduction in major cardiac events such as heart attack, stroke and death, which are the primary risks for patients whose blood pressure is not well controlled."
Renal denervation therapy is a minimally invasive procedure that uses radiofrequency (RF) energy to disrupt the renal nerves, which lead in and out of the kidneys. The RF energy creates lesions (tiny scars) along the renal sympathetic nerves – a network of nerves that help control blood pressure. This intentional disruption of the nerve supply causes systolic and diastolic blood pressure to decrease.
"Initial study results have demonstrated that the EnligHTN Renal Denervation System is safe and effective in rapidly lowering blood pressure. If these results extend into the prevention of major cardiac events, there is the potential to dramatically change how we treat these patients," said Professor Thomas Lüscher, chairman, cardiology and cardiovascular center at the University Hospital in Zurich, Switzerland, a principal investigator for the trial.
The EnligHTNment trial will be an international, multi-center, randomized, controlled study examining the safety and effectiveness of renal denervation with the EnligHTN™ Renal Denervation System in reducing risk of major cardiovascular events.
"We are committed to making the right investments to lead the emerging field of interventional treatment for hypertension," said Frank J. Callaghan, president of the St. Jude Medical Cardiovascular and Ablation Technologies Division. "Like other landmark trials we have sponsored, this first-of-its kind study provides the opportunity to evaluate patient outcomes that matter the most – heart attack, stroke and death."
St. Jude Medical is currently conducting two additional renal denervation studies called EnligHTN I and EnligHTN II that are evaluating the EnligHTN system for hypertension. Results to-date from the EnligHTN I trial demonstrated that patients with drug-resistant hypertension treated with the St. Jude Medical EnligHTN system had a rapid and sustained drop in blood pressure. After thirty days, systolic blood pressure was rapidly reduced by an average of 28 mmHg that remained stable with a reduction of 26 mmHg points six months after treatment. This is an important finding as the risk of cardiovascular death drops by half with every systolic decrease of 20 mmHg. The EnligHTN II trial is being conducted at 40 sites in Europe and Australia and will enroll approximately 500 patients with uncontrolled hypertension. The study began enrollment in January 2013 to evaluate the reduction in systolic blood pressure at six months across all enrolled patients after having renal denervation, and within sub-groups with varying degrees of kidney functionality.
About Renal Denervation and the EnligHTN System
Renal denervation is a catheter-based ablation procedure that potentially provides lasting reduction in blood pressure for patients with hypertension. A catheter is introduced through the femoral artery in the leg to access the renal arteries that connect to the kidneys. Once in place, the tip of the catheter is held against the surface of the artery where radiofrequency (RF) energy is delivered to the surrounding nerves.
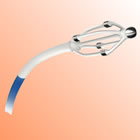
The EnligHTN system is a multi-electrode ablation technology that features a unique, non-occlusive basket design that delivers a predictable pattern of four evenly-spaced ablations with each catheter placement. This allows for continuous blood flow to the kidney during the procedure. Compared to single-electrode ablation systems, the multi-electrode EnligHTN system has the potential to improve consistency and save time, which may result in improved workflow and cost efficiencies.
In 2012, the EnligHTN Renal Denervation System earned European CE Mark approval and was launched in several markets. It is not yet approved for use in the U.S.
About St. Jude Medical
St. Jude Medical develops medical technology and services that focus on putting more control into the hands of those who treat cardiac, neurological and chronic pain patients worldwide. The company is dedicated to advancing the practice of medicine by reducing risk wherever possible and contributing to successful outcomes for every patient. St. Jude Medical is headquartered in St. Paul, Minn. and has four major focus areas that include: cardiac rhythm management, atrial fibrillation, cardiovascular and neuromodulation. For more information, please visit sjm.com.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, anticipated regulatory approvals and future product launches, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management's current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include market conditions and other factors beyond the Company's control and the risk factors and other cautionary statements described in the Company's filings with the SEC, including those described in the Risk Factors and Cautionary Statements sections of the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2011 and Quarterly Report on Form 10-Q for the fiscal quarter ended September 29, 2012. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.