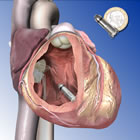
Both of these capsule-sized devices are 1/10 the size of a standard pacemaker and both are "leadless"; that is, they are designed to work directly inside the heart's chamber, eliminating the need for electrical wire leads, which have been the nexus for corrosion, complications, device failures and recalls. Nor do they require a surgical incision in the chest. They are delivered to the chamber of the heart by catheter via the body's circulatory system.
Not just limited to cardiac pacemakers, the miniaturization trend extends to cardiac monitoring devices: just last week Medtronic also announced the global launch of its Reveal LINQ Insertable Cardiac Monitor, 1/3 the size of a AAA battery.
The Reveal LINQ monitor has been approved for use in Europe and the U.S. Both St. Jude's and Medtronic's tiny pacemakers still are investigational devices in the U.S., although St. Jude's Nanostim gained CE Mark approval in October and is currently marketed in Europe.
The press release from Medtronic, Inc. about the first U.S. implant of the Micra follows:
Medtronic Announces First U.S. Implant of World's Smallest, Minimally Invasive Cardiac Pacemaker
Medtronic Enrolls First U.S. Patient in Global Clinical Trial for Miniature Transcatheter Pacemaker System
February 20, 2014 -- Minneapolis -- Continuing its leadership in advanced pacing technology and device miniaturization, Medtronic, Inc. (NYSE: MDT), today announced the first U.S. implant of the world's smallest pacemaker: the Micra™ Transcatheter Pacing System (TPS). The device was successfully implanted at NYU Langone Medical Center by Larry Chinitz, M.D., director of the Heart Rhythm Center at NYU Langone Medical Center in New York City, as part of the Medtronic global pivotal clinical trial. The Micra TPS is an investigational device worldwide.
At one-tenth the size of a conventional pacemaker, and comparable in size to a large vitamin, the Micra TPS is delivered directly into the heart through a catheter inserted in the femoral vein. Once positioned, the pacemaker is securely attached to the heart wall and can be repositioned or retrieved if needed. The miniature device does not require the use of wires, known as "leads," to connect to the heart. Attached to the heart via small tines, the pacemaker delivers electrical impulses that pace the heart through an electrode at the end of the device.
"With its small size and minimally invasive procedure, this technology represents the future of pacing," said Dr. Chinitz. "Eliminating the need for a lead and pocket has the potential to reduce complications and recovery times compared to traditional pacemaker implants, which would be a major benefit to patients."
In contrast to current pacemaker implant procedures, the Micra TPS implant does not require a surgical incision in the chest and the creation of a "pocket" under the skin. This eliminates a potential source of device-related complications, and any visible sign of the device.
"Micra TPS is an example of the significant investment we have made in disruptive technology, specifically the miniaturization of implantable cardiac devices," said Pat Mackin, president of the Cardiac Rhythm Disease Management business and senior vice president at Medtronic. "Less invasive, miniature device technologies show strong promise in improving patient outcomes and implant procedure efficiency. The FDA's interactive review with CRDM was a key part of the IDE application approval process, and through our global Micra TPS clinical trial, we intend to generate robust evidence of these benefits to patients and clinicians throughout the world."
Micra TPS Study Design
The study is a single-arm, multi-center global clinical trial that will enroll up to 780 patients at approximately 50 centers. Initial results from the first 60 patients, followed up to three months, are expected in the second half of 2014.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology - alleviating pain, restoring health, and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.