|
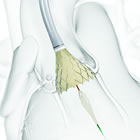
The first patients were enrolled at PinnacleHealth CardioVascular Institute in Harrisburg, PA. The heart team led by Mubashir Mumtaz, M.D., F.A.C.S., F.A.C.C. chief of cardiothoracic surgery and Hemal Gada, M.D., M.B.A., medical director of structural heart at PinnacleHealth performed the first procedures in the trial. "Our team of cardiologists and cardiovascular surgeons is pleased to be involved in this next phase of clinical investigation," said Dr. Mumtaz. "It is invigorating to be on the national forefront of care and to help heart teams understand the potential benefits of TAVR in a broader patient population." This clinical trial will include 1,200 patients with severe aortic stenosis who have a less than 3 percent risk of operative mortality, as determined by a heart team. The trial will enroll low-risk patients from up to 80 clinical sites with 1:1 randomization to receive the Evolut R System or undergo open-heart surgery (surgical aortic valve replacement or SAVR). The IDE is designed as an adaptive trial with a primary endpoint of all-cause mortality or disabling stroke. The trial has a 2-year endpoint and allows for a one-year analysis for early FDA submission. Additionally, the trial will include a sub-study of leaflet mobility in 400 patients. The Evolut R system is designed to increase conformability and sealing at the annulus, while maintaining supra-annular valve positioning for improved blood flow and hemodynamic performance. The valve is delivered through the EnVeo(TM) R Delivery Catheter System, which features an InLine Sheath that provides the lowest profile on the market (14 Fr equivalent, less than 1/5 inch). The low profile enables treatment of patients with vessels down to 5mm through the preferred transfemoral access route, and may minimize the risk of major vascular complications in some patients. The CoreValve Evolut R transcatheter valve and the CoreValve EnVeo R Delivery Catheter System were FDA-approved for commercial use in the United States in June 2015. It is also available in Europe and other countries that recognize the CE Mark. In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Source: Medtronic plc |