 |

|
 |

In
this interview, Angioplasty.Org talks with radialist Dr.
Samir Pancholy about his findings regarding the prevention
of radial artery occlusion, one of the few complications
associated with the transradial approach to catheter-based
procedures. Dr. Pancholy has authored or co-authored a number
of studies on aspects of transradial access, most recently
appearing in the Journal of Invasive Cardiology and Catheterization
and
Cardiovascular
Interventions. He has also been published in
the American Journal of Cardiology, the American Heart
Journal and the
Journal of Nuclear Cardiology, among others.
Dr. Pancholy is currently affiliated with Mercy Hospital
and Community Medical Center in Scranton, Pennsylvania, as
well as several other Scranton-area hospitals. He is considered
an expert in the transradial approach and has conducted courses
on this technique. He is board certified in Internal Medicine,
Nuclear Medicine and Interventional Cardiology.
He is a fellow of the Society for Cardiac Angiography and
Interventions and has served on SCAI's Credentials
Committee.
For more about the transradial approach, visit our Radial
Access Center.
|
|

Samir
B. Pancholy MD, FACC, FSCAI -- Mercy Hospital & Comm.
Medical Center, Scranton, PA |
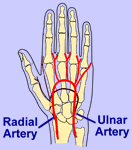
Q: What is radial artery occlusion?
Dr. Pancholy: Radial artery occlusion is basically obliteration of
the lumen of the radial artery (in the wrist) which becomes 100%
blocked. The most common reason is either traumatic or an occlusion
that occurs after a catheter-based procedure that uses the radial
artery, either catheterization or angioplasty. It is very infrequent,
but it remains one of the few complications of transradial procedures.
Q: What causes radial artery occlusion?
Dr. Pancholy: Surprisingly, until about a year ago, we did not
know the cause. We were hypothesizing as if we knew, but we had
no proof. We had a number of imaging studies, ultrasonography,
every once in a while contrast angiography, which would show
us that the radial artery was occluded, but we never really got
into the mechanism. There were lots of theories, such as growth
of extra tissue, scar growth which will then occlude the lumen
like it does in the coronary artery after we do angioplasty.
A lot of people thought, “Well clot’s got to be a
player. Blood clotting at the local area because of the trauma
to the artery is definitely a mechanism and then afterwards it
becomes organized into a scar.” But there was no proof
for this.
Q: Why was it so difficult to find
the cause of radial artery occlusion?
Dr. Pancholy: The reason there was no proof was because radial
occlusion is such a benign complication that nobody ever needs
to go to the operating room to get it fixed. So there had been
no direct look at the radial artery of a patient who had this
problem. The most we did was a sonogram and we found out the
artery was occluded, but the patient was asymptomatic and so
we used to tell them there's no harm done, you're going to be
fine.
Q: But you finally were able to determine the cause of radial
artery occlusion?
Yes. About a year ago here in Scranton, one of my patients had
no femoral artery access at all. Her left arm had a hemodialysis
fistula so I could not use the left radial or brachial artery,
and I had to use the right radial artery for the catheterization.
Turned out she had coronary blockages, so I was planning to go
back in and fix them, but I decided to wait a few days because
of her other issues.
When we brought her back,
we saw that the radial pulse was extremely diminished, which
was
brand-new because, when I had done the catheterization, she
had a nice right radial pulse. So we did a sonogram and found
out that the radial artery was occluded.
We now had two
options. We either go to the right brachial artery or we
somehow get back in through the occluded radial artery.
Brachial access
historically has been known to have the most complications
-- higher than femoral access and clearly higher than radial
access. So I decided to enter the radial artery distal
to the occlusion and I documented that with contrast. Once
I
knew
I was in the correct lumen, I put a wire into my needle
hub and gently advanced it -- and I was able to cross the
radial
occlusion. I was extremely happy that I was in the radial
artery, but when I put my sheath over that wire and took
out the dilator,
there was no blood flow. So I'm looking at it saying, my
goodness, I think I am in a false lumen. |
|

Samir
B. Pancholy MD,
FACC, FSCAI |
However, we applied some backward pressure through a syringe and
suddenly some blood came out of the sheath into my syringe and
the blood started flowing. We carefully retrieved the blood out
of the syringe without compromising it and I sent it to the pathology
lab -- and we were able to see under the microscope that it was
organizing thrombus with very rapid growth.
This was one of the first times that the pathologic mechanism
of radial artery occlusion was documented. It was actually thrombus
that should not have started organizing so fast, but we know that
it was thrombosis that goes on at the local spot and what our sheath
did was to dislodge the thrombus forward and then we sucked it
out with a syringe when we were aspirating and trying to re-establish
blood flow.
One month out from the angioplasty, we re-ultrasounded the radial
artery and it was wide open. I've been following her since August
of last year and she continues to stay open. In that series I had
14 patients in whom I was able to re-access 12, and the majority
of them stayed open.
Q: As you’ve written, although radial artery occlusion is
infrequent, it is the chief complication of the radial approach – benign
for the patient, but it may prevent re-use of the artery as an
access site for future procedures. So being able to use an occluded
radial artery for subsequent procedures is important?
Dr. Pancholy: Correct. And we published these findings in the December
2007 issue of the Journal of Invasive Cardiology (“Transradial
Access in an Occluded Radial Artery: New Technique”).
Q: So you verified thrombus as the cause of radial artery occlusion,
but what causes the thrombus and is there a way to reduce the risk?
Dr. Pancholy: Our hypothesis was that occlusive hemostasis was
the reason for radial artery occlusion. There was some proof to
that effect from previous retrospective databases: one small study
from Sanmartin in Spain showed that it was a potent predictor of
occlusion. So we thought, well, when a blood vessel gets traumatized,
for example during balloon angioplasty, what do we do to make sure
it doesn't clot up? One thing is to establish constant blood flow
so that the thrombus doesn't get a chance to propagate. So we thought
that occlusively compressing the radial artery to achieve hemostasis
was making chronic occlusion of the radial artery more frequent
than it would have been otherwise, just based on trauma.
Q: You tested this
concept in the PROPHET study, where your conclusion was that “Patent
hemostasis is highly effective in reducing radial artery occlusion
after radial access; guided compression should be performed
to maintain radial artery patency at the time of hemostasis,
to prevent future radial artery occlusion.”
Dr. Pancholy: Yes.
There's an abstract
online of the PROPHET study, and the full
study is going to be published in a forthcoming issue of Catheterization
and Cardiovascular Interventions. We wanted to test our theory
that if you don't compress the radial artery very hard, and
don’t occlude the flow through it during the process of hemostasis,
then you might actually lower the risk of occlusion -- and
this was what we called the concept of “patent hemostasis.” |
|
 |
Q: You’ve just recently completed a new study, a
summary of which we’ve posted at Angioplasty.Org. It’s similar
to the PROPHET study, except you’ve added a specific piece
of equipment to the mix: Terumo’s TR Band, to aid in achieving “patent
hemostasis” and “guided compression”. Tell us
more about both the PROPHET results and your new study.
Dr. Pancholy: At the time that PROPHET was started, we did not
have the TR Band in the U.S., so we were still using the old hemodialysis
band, called the HemoBand. And the HemoBand almost looks like a
zip tie that you essentially apply to the site of the sheath entry
and then just click it on and tighten up as you need to.
Because the radial artery is so isolated and against the bone,
it's very easy to apply a lot of pressure and not get into any
trouble. The patient tolerates it very well. They might feel a
little bit of pressure there. But it gives you very guaranteed
hemostasis. However, most operators I have observed actually want
to over-pressurize the radial site, once they take the sheath out,
just to be sure they don't get any bleeding.
With the PROPHET study, we took half of the patients and did our
standard pressure band application, and we randomized the other
half to get patency documented after the case, where we made sure
that we applied the band and then we liberated the pressure to
some extent until we got patency.
 |
|
Q: How were you able to
measure that you got patency?
Dr. Pancholy: We were able to
measure
it by doing plethysmography of the index finger. We applied
a pulse oximeter probe to the finger and we would occlude the
ulnar flow by applying direct pressure on it. We wanted to
see persistent flow going on in the radial artery which gives
you proof that the radial artery is widely patent at that time.
Most
of the time in the PROPHET study we would find that the radial
was occluded with our pressure band, so we would start loosening
the band,
and either we would get radial flow as evidenced by return
of the plethysmograph signal, or we would get bleeding, in
which case we would convert our hold to a manual hold. |
Q: The PROPHET study showed significant
reductions of radial occlusion, on the order of 60% at 24 hours,
for the group that had documented
patency and “guided compression”. What was the motivation
for adding the TR Band in for your new study?
Dr. Pancholy: In August of '07 the TR Band was approved by FDA
to be marketed in the U.S. As soon as it was available, we started
using it exclusively on everybody because it's much more comfortable
than the HemoBand. Obviously we had collected a lot of patients
who underwent transradial catheterization with the TR Band since
its availability, so we went back and looked at the occlusion data
on people who received HemoBand after the transradial cath vs.
those who received the TR Band after the cath and we found with
the TR Band a very significant reduction in the risk of occlusion
that follows these procedures, at 24 hours and at 30 days.
And it was surprisingly striking. So then we started wondering,
why are we having this difference? What's the mechanism of it?
We had actually been intrigued by the fact that the TR Band has
an inflatable chamber anyway. The HemoBand, once you apply it,
actually maintains the pressure that you applied initially for
the duration that you leave it on, unless you go to the HemoBand
and manually click it down to a lower pressure, or you essentially
untie the HemoBand.
| The TR Band, on the other
hand, has an inflatable chamber which we inflate with 15cc
of air according to the package insert. And what we noticed
was that the TR Band actually deflates over the first hour
or so. So we measured pressures in the chamber of the TR Band.
We were able to see that the initial pressure is usually around
the low 200's -- the mean was 240. And at half an hour that
pressure comes down to about 150, and at one hour it's about
130 or so -- without any intervention from us. So this is almost
a self-deflating band. What it does is to allow the radial
artery to go from an occlusive application to a non-occlusive
hold. And we did not see any bleeding complications from the
TR Band at all, so it wasn’t losing its efficacy as far being
a hemostatic device. |
|
TR Band
hemostasis device |
Q: Back to your study -- what was the magnitude of reduction of
radial artery occlusion using the TR Band?
Dr. Pancholy: Very, very significant. In our database which was
250 patients in each arm, we had about 55%-60% decrease in early
and chronic occlusion with the TR Band vs. the HemoBand, which
is a real big deal because occlusion is by far the only complication
of the transradial procedure that is considered to be worthwhile
as far as being concerning and one which limits future access.
Q: Does this method of using the
TR Band and deflating it take more time and can it be performed
by nursing staff?
Dr. Pancholy: It is automatic. The TR Band gives you an opportunity
to "keep your own protocol that you have now", and not
worry about occlusion as much as you would with other non-deflating
or non-releasing devices out there. We used to use the HemoBand
because it's the cheapest and most generic device -- it's probably
the most commonly-used device worldwide because it's so inexpensive,
but it comes with a price to pay, because you are not able to walk
away from the patient for two hours and get a low risk of occlusion
like you do with the TR Band.
Q: In your study, even after the reduction, the chronic radial
artery occlusion rate was 18 out of 250 -- that's still higher
than is quoted in some literature.
Dr. Pancholy: Yes. My rate of occlusion had been very low. But
that was before we brought every patient in for follow-up. After
we started examining every patient at 24 hours and 30 days, we
found that the actual occlusion rate was much higher, because it
is a totally asymptomatic kind of a complication, so you're not
ever going to get a patient come in and say I think I have an occlusion.
In my practice we have a constant 24 hour and 30 day follow-up
going on for radial artery occlusion for everybody that goes through
a transradial procedure. In fact we have radial weekends, where
on a Saturday morning, I call people into my office for bagels
and donuts, and we examine them for occlusion. It's a free visit
and they come and have a little breakfast and go home. This way
we get very tight data collection on every procedure. So if you
bring everybody back and look for a radial occlusion, you will
see more.
Q: So the take-away here is that
compression for too long at too high a pressure will lead to
an occlusion.
Would you say that the
idea of using “longer and stronger” compression is
a mind set coming over from people whose experience is with the
femoral approach, because in that situation you absolutely need
to use higher compression for a longer time? Could we say that:
"Long and strong in the radial is wrong"?
Dr. Pancholy: You're absolutely right. There is a mentality of
reinforcement and over-application and longer application from
the femoral experience and that actually is very counter-productive
in the wrist.
Q: You’re looking to establish
a protocol about this?
Dr. Pancholy: Yes. What all these data are trying to say is that
patency is a very big necessity when you are obtaining hemostasis
after a transradial procedure and systematic protocols for maintaining
patency need to exist. If you make an attempt at instituting
a patent hold, that may not be good enough. You have to document
it, you have to prove that you're creating patency, you have
to monitor it. And not use excessive pressure or excessive duration.
There is a difference between trying and protocolizing. And I
think we need to incorporate this as a protocol after the procedure,
not just an attempt. Because if it becomes a protocol, then everybody
follows it and it improves outcomes quite significantly.
This interview was conducted
in August 2008 by Burt Cohen of Angioplasty.Org.
|






