|
June 3, 2008 -- The question on the minds of a great many patients who have had a drug-eluting stent implanted is: "When can I stop taking Plavix? When will my drug-eluting stent be completely healed?" From John in the United Kingdom to H. Percell in Oregon to Patrick M. in Florida, the Patient Forums on Angioplasty.Org are filled with hundreds of postings asking this question.
But, until the development of OCT, there has been no direct way to see if struts have been covered, except through pathology specimens (it was in fact pathologist Renu Virmani who was one of the early voices of concern regarding the delayed healing around stents that she had seen in specimens from patients). And having a method to visualize whether the stent has "healed" may have significant implications for patients. When bare metal stents first arrived on the scene over ten years ago, the FDA required that patients be given dual antiplatelet therapy (aspirin plus clopidogrel, a.k.a. Plavix or ticlopidine, a.k.a. Ticlid) for 4-6 weeks. Studies showed that metal stents became covered by a protective layer of endothelial cells in this time period, reducing the risk of blood clotting. But in 20% of patients, tissue growth became so excessive that it blocked the blood flow through the stent, causing the very condition the stent was supposed to prevent. This phenomenon was called " in-stent restenosis". Enter the drug-eluting stent, designed to slow down the proliferation of endothelial cells and prevent them from aggregating too thickly. Because of the anti-proliferative properties of these medicated stents, the FDA more than tripled the length of antiplatelet therapy, allowing 3-6 months for the stent struts to become covered. However, in the fall of 2006, three years after U.S. approval, reports surfaced of increased late stent thrombosis (blood clotting inside the stent after six months) with drug-eluting stents. Although the percentage of patients affected was very low, less than 1%, stent thrombosis can cause heart attack or death more than a third of the time. Concerned about the public health implications, the FDA called a special two-day hearing and one result was that the major heart organizations changed their guidelines to a one-year minimum of dual antiplatelet therapy, assuming the patient does not experience bleeding complications or other adverse reactions. These guidelines were written as a "best estimate", because there have not been studies which conclude definitely the optimum duration of antiplatelet therapy. But there are several Catch-22s that many patients face. What if a patient does have adverse reactions to Plavix, either allergic rashes or bleeding complications? What if a patient needs surgery, such as a knee-replacement, and Plavix has to be stopped? What if a patient can't afford the $4/day cost of Plavix? What studies have shown is that early cessation of antiplatelet therapy in drug-eluting stent patients results in an increase in heart attack and death.
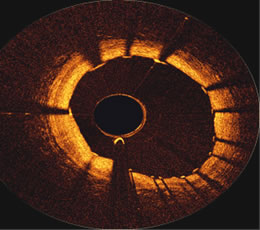
Like intravascular ultrasound (IVUS), OCT is a catheter-based system which is performed in a cath lab. Although IVUS can image to a greater depth into the artery wall, OCT provides a higher resolution of the inner artery surfaces (10 microns) and can easily show if stents have been covered. The ability to image the surface at high resolution also allows OCT to see very thin fibrous "caps" which are known to cover lipid-rich cores in the artery, so-called "vulnerable plaques". These plaques tend to be about 30 microns thick, so are easily identifiable using OCT. Identifying such areas may provide early detection of problems and help direct treatment for the prevention of heart attacks, especially in a system that may combine both OCT and IVUS technologies to give a more complete picture of the coronary artery. A study comparing IVUS and OCT in animal models has been published in the current issue of JACC Interventions, the new American College of Cardiology journal, devoted to interventional cardiology, and it concludes that OCT has the ability to visualize coverage of the stent. Furthermore, the importance of this imaging technology in determining the optimum duration of antiplatelet therapy is highlighted in an accompanying editorial, co-authored by Dr. Carlo Di Mario of Royal Brompton in London:
Optical Coherence Tomography is still investigational, but this new field is moving quickly. Less than a month after the JACC Interventions animal study was published, the First in Man Volcano OCT study commenced in Rotterdam. Results from these and other clinical work will be adding to the knowledge base and Volcano Corporation is hoping to have U.S. approval of their OCT technology sometime in the second half of 2009. For more information on OCT and other intravascular imaging and measurement technologies, visit the Intravascular Ultrasound Center on Angioplasty.Org.
Reported by Burt Cohen, June 3, 2008 (updated from May 29) |