|
Medtronic Renal Denervation Program Advances in U.S.
Symplicity Device Selected for FDA-CMS Parallel Review Program for Earlier Patient Access to Innovative Medical Advancements
Medtronic Submits IDE for 2nd U.S. Trial Studying Expanded Indications of 140-160mm Hg
|
 |
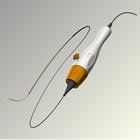
Symplicity™ Renal Denervation System |
March 7, 2013 -- Medtronic, Inc. (NYSE: MDT) has announced two landmarks in its quest to bring the first renal denervation system to market in the United States.
The Symplicity™ Renal Denervation System is currently available in Europe, along with several other renal denervation devices manufactured by St. Jude Medical, Covidien, Vessix and Recor.
Although thousands of patients have been treated with these new devices in Europe, Asia, Africa, Australia and The Americas, no renal denervation system is yet available in the U.S. In fact Medtronic is the only company currently running a pivotal clinical trial, aimed at FDA approval: the HTN-3 study which was begun in August 2011 and expects to complete enrollment this summer.
Follow-up for the HTN-3 trial is six months, so sometime in 2014, the outcomes from that trial should be presented. Only then will the FDA begin its review process for approval.
Concurrent Review Will Speed Availability
In the past, even though a new technology won FDA approval, it still took a significant amount of time for CMS (Medicare) to institute coverage, a determination that usually is mirrored by other insurers. And until a new device is covered by insurance, it may be approved, judged safe and effective, but in reality it still is not available to patients. Patients experienced this delay with such devices as the carotid stent -- the device was approved, but no insurance covered its use for some time, effectively delaying its entry into clinical practice.
However, a new and unique program has been developed by the U.S. government in which the FDA review is done concurrently with a determination of coverage by CMS. The Symplicity System has been chosen as one of the first medical devices to be accepted into this program so, if and when it is approved, it will also be immediately available for patients throughout the U.S.
Expanded Trial Announced
Medtronic has also announced a new randomized clinical trial, the HTN-4, which will study the Symplicity™ renal denervation system for the treatment of uncontrolled hypertension in patients with systolic blood pressure between 140-160 mm Hg despite treatment with three or more anti-hypertensive medications of different classes. (The earlier HTN-3 looks at those patients with > 160mm Hg.)
These trials, along with Medtronic's global clinical evaluation program, will be looking at 8,000 patients for safety and efficacy. The desired result is to stabilize and lower the blood pressure of those patients in whom medications are not working sufficiently. An interventional procedure may be able to release these patients from medical therapy entirely. Furthermore, because hypertension is so closely linked with cardiovascular events, heart attack, stroke, heart failure, etc. there may be a mortality benefit, as well. To that end, Medtronic plans to follow this large global patient cohort for five years.
Two press releases from Medtronic, Inc. follow:

Symplicity™ Renal Denervation System One of First Devices Accepted to Participate in Concurrent Review for Joint FDA Premarket Approval and Medicare National Coverage Determination
FDA-CMS Parallel Review Program Designed to Enable Efficient and Earlier Patient Access to Innovative Medical Advancements
March 6, 2013 -- Minneapolis -- Medtronic, Inc. (NYSE: MDT) today announced the U.S. Food and Drug Administration (FDA) and the Centers for Medicare & Medicaid Services (CMS) have accepted the inclusion of the Symplicity™ renal denervation system for treatment-resistant hypertension in their parallel review program, which will allow CMS to begin consideration for national coverage determination while the FDA completes its review of safety and efficacy. The Symplicity renal denervation system currently is only available for investigational use in the United States. The Symplicity renal denervation system is one of the first medical devices to participate in the pilot program for concurrent review designed to facilitate the development of innovative new products and increase the efficiency of the review processes for both agencies. The two federal agencies accepted the Symplicity renal denervation system into the parallel review pilot program through a selection process that is limited to a few innovative devices per year.
The parallel review will be based primarily on results from Symplicity HTN-3, Medtronic's U.S. clinical trial of the Symplicity renal denervation system for treatment-resistant hypertension. In August 2011, the FDA approved Symplicity HTN-3, allowing Medtronic to become the first company to conduct a randomized, controlled trial of renal denervation in the U.S. Enrollment in this study is ongoing expected to be complete by the summer of 2013.
"We are pleased that FDA and CMS have accepted the Symplicity renal denervation system for parallel review. This joint review represents a significant step forward in accelerating patient access to renal denervation in the U.S.," said Sean Salmon, Senior Vice President and President, Coronary and Renal Denervation, Medtronic. "We look forward to working with both agencies to ensure an efficient and timely review so that we may offer a new treatment option for the millions of people with treatment-resistant hypertension in the U.S."
Symplicity HTN-3 is a single-blind, randomized, controlled trial designed to evaluate the safety and effectiveness of renal denervation with the Symplicity renal denervation system in patients with drug-resistant hypertension. The study will randomize 530 patients in up to 90 U.S. medical centers; patients in the clinical trial will be randomized to receive either renal denervation and treatment with anti-hypertensive medications or treatment with anti-hypertensive medications alone. The primary endpoints of the study are the change in blood pressure from baseline to six months following randomization and incidence of major adverse events one month following randomization. More information about Symplicity HTN-3 can be found at www.symplifybptrial.com.
Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. The nerves passing to the kidneys are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels.
About Treatment-Resistant Hypertension
Treatment-resistant hypertension, defined as persistently high blood pressure despite three or more anti-hypertensive medications of different types including a diuretic, puts approximately 120 million people worldwide at risk of premature death from kidney disease and cardiovascular events such as stroke, heart attack and heart failure. [1] [2] Research suggests that nearly one third of treated hypertensive individuals are considered resistant to treatment. [3]
About the Symplicity™ Renal Denervation System
The Symplicity renal denervation system's catheter and proprietary generator and algorithms were carefully and specifically developed through years of clinical experience to accomplish the renal denervation procedure. The Symplicity renal denervation system was launched commercially in April 2010 and is currently available in parts of Europe, Asia, Africa, Australia, Canada and Latin America and has been used to treat thousands of patients with hypertension worldwide.
The Symplicity renal denervation system consists of a flexible catheter and proprietary generator. In an endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity™ catheter into the femoral artery in the upper thigh and threads it into both renal arteries in turn. Once the catheter tip is in place within the renal artery, the Symplicity™ generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm aiming to deactivate the surrounding renal nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology – alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Symplicity is a trademark of Medtronic Inc. and is registered in one or more countries of the world.
[1] Egan, Brent M., et al. "Uncontrolled and Apparent Treatment Resistant Hypertension in the United States, 1988-2008."Circulation 2011;124:1046-1058.
[2] Hypertension and cardiovascular disease. World Heart Federation. 2011. http://www.world-heart-federation.org/ cardiovascular-health/cardiovascular- disease-risk-factors/hypertension/. Accessed January 23, 2012
[3] Doumas, Michael, et al. "Benefits from Treatment and Control of Patients with Resistant Hypertension." International Journal of Hypertension 2011 (2011) Article ID 318549, 8 pages, 2011. doi:10.4061/2011/318549.

Medtronic Submits IDE to FDA for U.S. Randomized Clinical Trial for Uncontrolled Hypertension Patients with Systolic Blood Pressure Between 140-160 mm Hg
IDE Submission for Symplicity HTN-4 Expands the Indicated Patient Population and Builds upon Medtronic's Rigorous Global Clinical Trial Program for Hypertension Treatment
March 7, 2013 -- Minneapolis -- Medtronic, Inc. (NYSE: MDT), announced today that the company has submitted an Investigational Device Exemption (IDE) to the U.S. Food and Drug Administration (FDA) to study the Symplicity™ renal denervation system for the treatment of uncontrolled hypertension in patients with systolic blood pressure between 140-160 mm Hg despite treatment with three or more anti-hypertensive medications of different classes. The Symplicity renal denervation system currently is only available for investigational use in the United States. Symplicity HTN-4 is the next step in Medtronic's global renal denervation clinical program designed to carefully and progressively build the clinical evidence platform for the treatment of hypertension. This is being accomplished through a series of trials, which include more than 250 patients already enrolled in the Symplicity HTN-1 and Symplicity HTN-2 studies, 530 patients being enrolled in the Symplicity HTN-3 study, and 5,000 patients being enrolled in the Global SYMPLICITY Registry. The robust SYMPLICITY global dataset with planned follow-up to 5 years in most studies will be utilized to evaluate not only blood pressure reduction, but also long-term cardiovascular outcomes from hypertension such as stroke, myocardial infarction, heart failure and cardiovascular death. Medtronic's rigorous clinical evaluation program of the Symplicity renal denervation system includes more than 8,000 patients worldwide, including studies in the U.S., China, and Japan, with over 1,200 of these patients participating in randomized clinical trials.
The Symplicity HTN-4 trial will be the company's second randomized clinical trial in the U.S., with a patient population in line with the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7), the American Heart Association and the European Society of Hypertension definition of resistant hypertension. Symplicity HTN-4 builds upon the Symplicity HTN-3 study, Medtronic's pivotal U.S. clinical trial of the Symplicity renal denervation system for the treatment-resistant hypertension with systolic blood pressure greater than 160 mm Hg. Symplicity HTN-3 is the only clinical trial to date to receive an IDE approval to study renal denervation in the U.S. Medtronic aims to begin enrolling patients in the Symplicity HTN-4 trial in the second half of 2013, pending regulatory approval.
The principal investigators of Symplicity HTN-4 are David Kandzari, M.D., Director and Chief Scientific Officer, Interventional Cardiology and Interventional Cardiology Research, Piedmont Heart Institute, Atlanta, GA, and Michael Weber, M.D., Professor of Medicine, Division of Cardiovascular Medicine, at the SUNY Downstate College of Medicine in Brooklyn, New York.
"Symplicity HTN-4 demonstrates Medtronic's commitment to providing randomized safety and efficacy data for renal denervation in a wide variety of patients, as well as help increase our understanding of the potential benefit of renal denervation on patients with a less severe form of treatment-resistant hypertension," said Sean Salmon, Senior Vice President and President, Coronary & Renal Denervation, Medtronic. "We intend to continue to add to the substantial body of evidence Medtronic has generated to support the use of renal denervation in broader patient populations worldwide in conditions associated with hyperactive sympathetic nervous system drive, including uncontrolled and treatment-resistant hypertension, and heart failure."
Hypertension is a major and growing global public health concern. It affects an estimated 30–40 percent of the adult population1-3 and contributes directly to strokes, heart attacks, heart failure and cardiovascular mortality.4, 5 Numerous studies have demonstrated the continuous and consistent benefit in terms of risk reduction with controlling blood pressure.4-6 Despite the availability of numerous safe pharmacological therapies, the percentage of hypertensive patients achieving blood pressure control to guideline target blood pressure values remains low.
In the United States, the control rate for hypertensive patients taking medications is approximately 60 percent,7 leaving many uncontrolled hypertension patients at an increased cardiovascular risk. Many patients with uncontrolled hypertension meet the criteria for treatment resistant hypertension in that their systolic blood pressure is = 140 mm Hg despite being on three or more anti-hypertensive medications. The risk inherent with persistently high blood pressure in these patients warrants special therapeutic considerations.8
Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that lie within the renal artery wall and lead into and out of the kidneys. The nerves passing to the kidneys are part of the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, the heart, the kidneys and the blood vessels.
The Symplicity system's catheter and proprietary generator and algorithms were carefully and specifically developed through years of clinical experience to accomplish the renal denervation procedure. The Symplicity renal denervation system was launched commercially in April 2010 and is currently available in parts of Europe, Asia, Africa, Australia, Canada and Latin America and has been used to treat thousands of patients with treatment-resistant hypertension worldwide.
The Symplicity renal denervation system consists of a flexible catheter and proprietary generator. In an endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity™ catheter into the femoral artery in the upper thigh and threads it into both renal arteries in turn. Once the catheter tip is in place within the renal artery, the Symplicity™ generator is activated to deliver a controlled, low-power radio-frequency (RF) energy routine according to a proprietary algorithm aiming to deactivate the surrounding renal nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is an established contributor to chronic hypertension. The procedure does not involve a permanent implant.
The FDA granted Medtronic approval for the Symplicity HTN-3 study in August 2011. More information about Symplicity HTN-3, which is currently enrolling patients, can be found at www.symplifybptrial.com.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology – alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Symplicity is a trademark of Medtronic Inc. and is registered in one or more countries of the world.
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2-e220
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913
- Lawes CM, Rodgers A, Bennett DA, Parag V, Suh I, Ueshima H, MacMahon S, Asia Pacific Cohort Studies C. Blood pressure and cardiovascular disease in the asia pacific region. Journal of hypertension. 2003;21:707-716
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252
- Staessen JA, Li Y, Thijs L, Wang JG. Blood pressure reduction and cardiovascular prevention: An update including the 2003-2004 secondary prevention trials. Hypertension research : official journal of the Japanese Society of Hypertension. 2005;28:385-407
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J, National High Blood Pressure Education Program Coordinating C. Primary prevention of hypertension: Clinical and public health advisory from the national high blood pressure education program. JAMA : the journal of the American Medical Association. 2002;288:1882-1888
- Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among united states adults with hypertension: The national health and nutrition examination survey, 2001 to 2010. Circulation. 2012;126:2105-2114
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM, American Heart Association Professional Education C. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the american heart association professional education committee of the council for high blood pressure research. Circulation. 2008;117:e510-5
Reported by Burt Cohen, March 7, 2013
|

