![]()
<< To Homepage >>
<<Archives>>
January 2009 Archives: January 30, 2009 -- 9:50am EST Wrist Angioplasty Gaining Acceptance in
U.S. A big question is why it isn't used more in the U.S. -- in Europe, Asia and South America it's done far more often (40-50% as opposed to 2%) than the "standard" femoral approach which uses an artery in the groin. One answer is training and interest. Traditionally cardiologists aren't trained to do radial procedures during their fellowships. Another is the perception that certain complex interventional procedures are more difficult to do via the wrist. But this may be changing. Yesterday Dr. John Coppola of St. Vincent's Hospital in New York held one of his radial courses -- and it did not need to be promoted, because it was filled to capacity even before being announced. Terumo Interventional, which makes equipment specifically for the radial technique, told me that all the training courses in the U.S. that they are involved in currently have a waiting list -- this certainly was not the case only a year ago. This past fall major meetings like TCT and ISET have been featuring symposia on the radial technique, and a recent article, "Radial-access PCI safe and successful in high-risk patients and complex lesions", published on theheart.org, generated far more online comments from cardiologists than the average story. And a recent study of 600,000 patients, published in JACC Interventions, showed a sharp uptick in radial procedures in the last quarter of 2007. (More current data is not yet available.) And the popular press has been reporting more as well: the CBS "Early Show" recently did a two-minute segment, with Dr. Howard Cohen sitting on a park bench showing catheters to host Julie Chen! So all of these movements are showing a gain for the wrist approach in the U.S. One big reason, of course, is that results show lower bleeding complications, and lower mortality with the wrist approach: a safer approach to both angioplasty and diagnostic cardiac catheterization. Support for these views can be found in the many peer-reviewed journal articles listed in the Reference and Bibliography section of Angioplasty.Org's Radial Access Center (now in its second year). But one such study, published in JACC back in 2005, recently was brought to my attention. A Stanford study of 3,500 consecutive patients undergoing angioplasty showed the incidence of retroperitoneal hematoma (RPH) at 0.74%. This is a very serious complication, where bleeding occurs at the femoral access site, but backwards into the large abdominal space in the body. It's often not recognized until well after the procedure and it can be very dangerous, resulting in serious blood loss, increasing morbidity and mortality. This means 1 in 135 patients will suffer this complication. And it was significantly more common in women. The radial approach virtually eliminates this complication. Stay tuned.... « permalink » « send comment » « back to top »
January 28, 2009 -- 10:25am EST Plavix Safety Update So here is a link to the paper, titled: "ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use". The summary states:
« permalink » « send comment » « back to top »
January 26, 2009 -- 2:30pm EST Plavix, Prilosec, Prevacid, Protonix --
Do I Hear a Nexium? The basic concern is that some recent studies have questioned whether the effectiveness of Plavix may be compromised by a group of drugs known as Proton Pump Inhibitors (PPI). Presented as an abstract at the AHA in November, a major study by Medco, the nation's leading pharmacy benefit manager, looked at almost 17,000 patient benefit records and found a 74% increase in heart attacks in patients taking both Plavix and PPIs together. These include the drugs in my title, plus a few more. (Compounding the concern is that Prilosec is also available over-the-counter.) PPIs are often prescribed for patients who are taking Plavix, because Plavix, especially when taken with aspirin, can cause upset stomach and gastric bleeding and these PPIs are effective in reducing those adverse reactions. The problem is that PPIs may also inhibit the enzyme that activates clopidogrel. While some studies have shown this to be true, others have not. Also, this is not the first time that Proton Pump Inhibitors have come under review by the FDA. In August 2007, the FDA started a review, based on some small studies showing an increase in heart problems. But the clopidogrel connection was not involved there. One of the problems is that all drug eluting stent patients are required to take aspirin and clopidogrel for a year, at least. Stopping prematurely can lead to stent thrombosis (blood clots in the stent) and heart attack or death. So, if a drug is found to inhibit Plavix, it could be dangerous. However, the CREDO study, also presented at this year's AHA, found that there was no interaction, that patients taking PPIs showed an increase in cardiovascular events at one-year whether the patient was on Plavix or not. And that Plavix had a beneficial effect in reducing cardiovascular events whether the patient was taking PPIs or not. Confused? The conflicting results between these two major studies prompted the AHA, ACC and American College of Gastroenterology to issue a statement to patients not change their medications without consulting their clinician. The SCAI also issued a similar statement, and concluded by stating:
Unfortunately, Cogentus Pharmaceuticals, the company sponsoring the COGENT-1 trial announced on Thursday that it is filing for bankruptcy and that the trial was being terminated. Still confused? Wait....other recent studies have also shown various genetic markers which indicate not all patients process Plavix efficiently -- this is not news to those who have been discussing "Plavix resistance" in some patients for some time. So many questions about a drug that every stent patient must take! In today's notice, the FDA recommends:
« permalink » « send comment » « back to top »
January 25, 2009 -- 5:30pm EST Medtronic Seeks Runners -- Stents Included I am always amazed at the number of people who continue their athletic activities after having a device implanted (most could outrun me with no difficulty -- but that's another story...). Our Forum Topic, "Exercise, Sport, Physical Activity After Stent", has the stories of scores of stent and heart patients who continue to run, ride horses, etc. (By the way, I confirmed with Medtronic that patients who have coronary stents are eligible for the competition -- as long as there is no concurrent untreated coronary disease. Individuals with abdominal aortic stent grafts are not eligible.) More from Medtronic's press release:
To apply or recommend someone to be a 2009 Medtronic Global Hero, visit medtronic.com/globalheroes. Good luck! « permalink » « send comment » « back to top »
January 21, 2009 -- 12:20pm EST XIENCE V™ Stent Takes the Lead
The XIENCE V was approved by the FDA in July and is part of the new generation of stent technology, which I characterized last May as "DES 2.0", composed not just of new stent technologies, like XIENCE V and Medtronic's Endeavor, but a new way of looking at the process of stenting: when is it beneficial?, how to do it better?, how to verify proper placement?, etc. Interestingly enough, another company involved in DES 2.0 is Volcano. The company doesn't make stents, but innovates in imaging technologies like IVUS, OCT and Volcano also has been reporting double-digit growth, recently reporting a boost in sales of its FFR functional measurement products, due in great part to the striking results from the FAME trial. It seems that FFR (or Fractional Flow Reserve) is becoming a hot property in its own right -- St. Jude Medical just acquired Radi Systems, the other manufacturer of FFR devices. And more innovations will be coming: Abbott and others are working on biodegradable stents that will completely disappear when their work is done, Cardiac CT has made significant advances in lowering the radiation dose without compromising image quality and, in the near future, may be incorporating the ability to perform perfusion measurement, currently the territory of the nuclear stress test. Clearly the word for the future in healthcare is cost-effectiveness (well, okay, it's a hyphenated word...). But new technologies are going to have to prove that they can reduce costs AND benefit patient care. Of course, any advance in patient care will have the effect of reducing costs down the road (less repeat procedures, less care needed for chronic conditions, etc.). Whether innovation is enough to turn the economic tide, at least in the device industry, is yet to be seen, but these recent figures certainly are good news. « permalink » « send comment » « back to top »
January 19, 2009 -- 7:20pm EST When Less is More: Stents, That Is Yes, the FAME study did show that when FFR (Fractional Flow Reserve) was used routinely to measure whether or not a coronary lesion was ischemic, less stents were implanted per patient, and the resulting decrease in major cardiac events was significant. But stenting, as an important therapy, was never called into question. The patient population for the FAME study consisted of patients with multivessel disease -- and 94% of these patients had stents implanted. Yes, that's 94%! It's just that the number of stents per patient was lower, on the average: two stents instead of three.
He characterized the FAME results as a refinement of stenting, and that they actually may expand the use of the procedure as well. You can read Angioplasty.Org's exclusive interview with Dr. Pijls here. « permalink » « send comment » « back to top »
January 18, 2009 -- 11:30am EST Glad to be of Service!
The reader, by the way, had a heart attack a year-and-a-half ago, and two bare-metal stents inserted in a very critical artery to prevent "another coronary event". He also made lifestyle changes and feels better today than he has in years. He also continues reading the New York Times.
« permalink » « send comment » « back to top »
January 17, 2009 -- 7:30pm EST FAME: "Back to the Future" Well...it's not a new device; it's definitely not a new concept; and St. Jude doesn't exactly get the credit for it either, since their chief role was to acquire the company that created the product. But it is an important study! First off, read Angioplasty.Org's article on the FAME study, which clarifies some of this; then read my exclusive interview with the FAME study's co-principal investigator, Dr. Nico Pijls.
Gruentzig continually monitored the pressure gradient during procedures and, when the distal and proximal pressures were similar, due to the dilatation or expanding of the balloon and compression of the plaque, he judged the procedure finished. Optimal dilatation of the stenosis had been achieved! Gruentzig was also very conservative regarding the ability of angioplasty to achieve a result. He called dilatation "a controlled injury" of the artery and was adamant that the decision to intervene needed to be made with great care and understanding of the downsides of the procedure. In these early days, the measurement of pressures required a separate lumen, which made the balloons were pretty wide and unable to get into narrow arterial spaces. Then Dr. John Simpson invented a much thinner balloon catheter: he got into the narrow spaces, but he sacrificed the ability to measure pressures. Gruentzig was not entirely comfortable with this "advance". But unfortunately he died in a plane crash in 1985, and well.... Fast-forward to the 21st century. For two decades, angioplasty has been done without monitoring the procedure with pressure gradients. Decisions were made primarily by eye (a.k.a. the oculo-stenotic reflex): there's a narrowing there; let's put in a stent. Now, thanks to Dr. Nico Pijls and others, cardiologists can measure pressures with a guide wire, using Doppler sensors et al. We are now able to measure gradients and make intelligent, data-based decisions about whether or not to dilate a blockage. The results? Well, don't intervene on a lesion/blockage that is not significant. It may do more harm than good. Gruentzig would have said the same. And now the FAME study reaffirms what he already knew, but which has been forgotten through the years. Back to the Future! « permalink » « send comment » « back to top »
January 15, 2009 -- 8:10pm EST Thank You, Associated Press, for the Too
Many Stents Headline Dr. Pijls hoped this wasn't the case, but I was pretty confident that it would be. However, yesterday I became a bit concerned, because most of the popular press seemed to report this results of this trial fairly accurately -- that is, until the Associated Press article came out. "Fewer clogged arteries may need stent treatment" is the AP's dreadline, and that kind of sets up the article. I mean if I had a clogged artery, I would want it opened. Of course, the FAME trial in no way suggests that a "clogged artery" should not be stented. The FAME study is about arteries that show up as having a blockage, but they are not significant blockages. Any artery that is "clogged", should be opened. But these are symantics, and the AP is interested in...well here's the opening sentence: "A new study gives fresh evidence that many people with clogged heart arteries are being overtreated with stents". Overtreated -- once again implying that there are cardiologists who can't wait to stick a stent in your artery. The study results also were reported by KABC in Los Angeles thus: Study: Stents could be harmful. For a rational explication of the FAME study results, check out our exclusive interview with Dr. Pijls. But I welcome the AP article, because now I won my bet. « permalink » « send comment » « back to top »
January 13, 2009 -- 4:45pm EST Dreadlines About Stents Revisited So only days after the NEJM article, the New York Times published a piece by esteemed health reporter Jane Brody, titled "More Isn’t Always Better in Coronary Care". And in case you were wondering where this article was headed, you might just take the lead sentence:
Amok, as defined by the American Heritage Dictionary:
Yow! Got the image? Hordes of out-of-control invasive cardiologists running around, crazed and sticking stents into patients' arteries with neither rhyme nor reason: killer cardiologists!! If Ms. Brody was not envisioning such a dramatic picture, perhaps she should have used a more accurate term -- however, her article certainly delivers the message that angioplasty, stenting and interventional cardiology in general are being grossly over-used: an assumption that is not only untrue, but dangerous!! Dangerous? How can a mere article in the "newspaper of record" be dangerous? Is the pen mightier than the catheter? Is there such a thing as a "killer journalist"? My answer is "yes". And here is why. Two years ago, the CHARISMA study was presented at the American College of Cardiology. The study's take-home message was that aspirin plus Plavix did not add benefit, and that in a subset of patients, it slightly increased heart attacks and mortality. But the study had nothing to do with stent patients. If you had a stent, it was critical that you continued to take both aspirin and Plavix. I wrote about this extensively and issued a warning to stent patients "Don't Stop Taking Your Meds". But patients read the popular press headlines (or rather the "dreadlines") which totally misreported the study and stated: "Plavix plus aspirin may be a risky combination", "Plavix with aspirin is deadly for some", and so on. And patients did stop taking their meds. And in the next couple of weeks, patients with stents wound up having heart attacks. I know this to be true, verified by cardiologists I have spoken to. So reading (or seeing) the news can cause heart attacks! That being said, I would also like to state that nowhere in Jane Brody's brief 800-word story is the issue of emergency angioplasty mentioned. Ms. Brody and the popular press have been delivering the message for quite a while now that angioplasty and stents are overused and that you should do everything you can to avoid them. There's only one catch: if you are having a heart attack, you need to get to a cath lab ASAP so that a balloon can open up your blocked artery and prevent your heart muscle from dying. 90 minutes is what you should aim for. This is not hype; this is fact: backed up by multiple studies over the past two decades. Angioplasty saves heart muscle. But the retail press would have you believe otherwise. As Dr. Gregory Dehmer, former president of the SCAI, told me a few days ago:
"It doesn't work". Thank you Jane Brody. Her article goes into more detail about the types of plaque that cause heart attacks, etc. and it leaves you with the sense that doctors don't really know what they're talking about. But her article depends almost entirely on the observations of one single physician: Dr. Michael Ozner, author of the book, "The Great American Heart Hoax". His idea, that more resources should be put into prevention, is completely correct. But his statement that:
is misleading. It is true that interventional cardiology is not treating the underlying biology of the arteries, but it is possibly preventing an acute event in those arteries -- one that just might save your life or, at least, save your heart muscle. There have been many recent studies that have proven this to be true. Yet Brody's article quotes decade-old studies to make her point -- a point which is, in fact, misleading at best. « permalink » « send comment » « back to top »
January 1, 2009 -- 8:40pm EST New England Journal Criticizes CBS News
on Transradial Angioplasty Report A leading thesis of the article is that journalists need to be aware that their reports can influence the behavior of clinicians and patients -- and on that point we agree completely. After all, one of the significant tasks that Angioplasty.Org has taken on over the years has been to correct misleading news stories about the field of interventional cardiology. Recent important examples include the flawed coverage of both the CHARISMA and COURAGE trials. We also are very aware that many patients and healthcare professionals respond to these "retail press" stories by going to search engines to delve further. For example, traffic on Angioplasty.Org spikes every time a major story about stents or angioplasty hits the newswires. Moreover, we agree with Ms. Dentzer that the popular press often will characterize the results of a study incorrectly, in order to concoct what we have dubbed a "dreadline". And these scare headlines have consequences. Cardiologists we have interviewed confirm that in March of 2006 some stent patients stopped taking Plavix and aspirin together, based on faulty headlines about the CHARISMA trial, and subsequently suffered heart attacks. There is a definite danger in misreporting -- and for that we applaud Ms. Dentzer's article. But in her article, she cites a recent 105-second TV segment about the transradial approach to angioplasty that aired on CBS's "Early Show" and she takes it to task for incorrect reporting. Since we host the major online source of information in the U.S. about the transradial approach, and since we feature an interview with Dr. Howard Cohen, the subject of the CBS News piece, and since we are also aware that CBS News logged onto our site in the week preceding their report to research the issue, we must take issue with the New England Journal critique of this piece. We, in fact, were impressed with the accuracy of the reporting by interviewer Julie Chen. Moreover, since I featured the CBS report in this blog, I feel obligated to defend my choice. Ms. Dentzer critiques:
But she is misreading the inflection that reporter Julie Chen used in introducing the piece. The transcripts reads:
Ms. Chen was very specifically drawing attention to the fact that the wrist approach was not the norm for angioplasties -- which was the entire point of this very short piece. Her second question to Dr. Cohen clarifies this:
Other criticisms that the NEJM article levels at CBS is that, although Dr. Cohen states the wrist approach is cheaper, he is not given time to "to cite the study on which his assertions |
 Over
the past year,
Over
the past year,  Medtronic
(NYSE: MDT) recently
Medtronic
(NYSE: MDT) recently 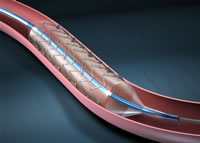
 In
fact Nico Pijls, the co-principal investigator for FAME, told Angioplasty.Org
that, rather than reduce the total number of stents used, the use
of FFR could easily expand the patient population that could benefit
by stents. For example, patients with multiple blockages might only
have only 2 that are measured as ischemic, and thus would be candidates
for stenting, rather than surgery.
In
fact Nico Pijls, the co-principal investigator for FAME, told Angioplasty.Org
that, rather than reduce the total number of stents used, the use
of FFR could easily expand the patient population that could benefit
by stents. For example, patients with multiple blockages might only
have only 2 that are measured as ischemic, and thus would be candidates
for stenting, rather than surgery.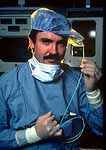 OK.
Now, let's get in the time machine and return to the genesis of coronary
angioplasty. It's 1977 and Andreas Gruentzig has fashioned a balloon
catheter to open a blockage in a patient's coronary artery. The balloon,
which he is holding in the picture to the left, not only can open
a blockage in a coronary artery but, oddly enough, also has the capability
of measuring the blood flow or pressure at the proximal and distal
ends of the stenosis (a.k.a. "blockage"). If the blockage
is significant, the two pressures are widely divergent, demonstrating
that there is a diminished blood flow through this arterial segment.
These "pressure gradients" would be displayed on the monitor
behind him, if there were an actual procedure going on.
OK.
Now, let's get in the time machine and return to the genesis of coronary
angioplasty. It's 1977 and Andreas Gruentzig has fashioned a balloon
catheter to open a blockage in a patient's coronary artery. The balloon,
which he is holding in the picture to the left, not only can open
a blockage in a coronary artery but, oddly enough, also has the capability
of measuring the blood flow or pressure at the proximal and distal
ends of the stenosis (a.k.a. "blockage"). If the blockage
is significant, the two pressures are widely divergent, demonstrating
that there is a diminished blood flow through this arterial segment.
These "pressure gradients" would be displayed on the monitor
behind him, if there were an actual procedure going on. "Right
after the COURAGE Trial was published, and this is not the fault
of the investigators of the COURAGE Trial -- it's the fault of
the way the media rolled the story out -- because I remember
watching the 5 O' Clock national news and the headline was "Angioplasty
Doesn't Work".
"Right
after the COURAGE Trial was published, and this is not the fault
of the investigators of the COURAGE Trial -- it's the fault of
the way the media rolled the story out -- because I remember
watching the 5 O' Clock national news and the headline was "Angioplasty
Doesn't Work". As
Editor-in-Chief of the most popular public website devoted to
interventional cardiology, I approached Susan Dentzer's article,
As
Editor-in-Chief of the most popular public website devoted to
interventional cardiology, I approached Susan Dentzer's article,