![]()
<< To Homepage >>
<<Archives>>
June-August 2009 Archives: August 22, 2009 -- 3:10pm PDT Drug-Eluting Stents: Looking Back
Quite so. After all, DES usage (at the time a $5 billion per year business) fell from 90% to 58% in a matter of months. Financial analysts were quite fixated on documenting those numbers. As were the discussions during subsequent cardiology meetings, such as the October 2006 TCT (see "Stent Wars Across the Atlantic: USA vs. Europe") reaching a peak in December at a special two-day Stent Safety Panel, convened by the FDA. I remember sitting in the hotel conference room in Gaithersburg, watching PowerPoint after PowerPoint about drug-eluting stent trial results, listening to the Swedish cardiologists present the SCAAR study which significantly ramped up fears about stent thrombosis, anecdotally referring to DES as the "death stent". I watched the executives and medical directors of the stent manufacturers being grilled by the FDA panel and saw panel members' incredulous reactions to the fact that there really wasn't very good data on long-term use of antiplatelet therapy or on "off-label" indications. I listened to the constant clacking of keyboards, as reporters from NBC, Wall Street Journal, New York Times instantly launched the speakers' words into cyberspace. I also testified to the panel on behalf of patients who were confused and scared about the "tiny time bombs" in their hearts. And I remember stopping Daniel Schultz, head of FDA's device division, in the parking lot and thanking him for convening this important meeting. So much for fond remembrances. Two weeks ago Daniel Schultz announced he was resigning his position "by mutual agreement". He'd been criticized for being too close to industry and with the change that's occurred in Washington, the FDA and...well, thank you very much, time to be on my way. (BTW, my observation at the 2006 Stent Safety Panel from the looks on the faces of the various industry execs, was that this had not been a happy pro-industry session!). However, some of the questions raised then have been answered (others not, but that's another column). The Swedish SCAAR Registry team totally reversed itself a year later (as predicted by Marty Leon and Gregg Stone). When longer time periods and more data were analyzed, there was absolutely no difference in mortality between drug-eluting and bare metal stents. (Sorry 'bout that!) Since the 2006 firestorm, two new 2nd generation drug-eluting stents have been introduced to the U.S. market (Abbott's XIENCE and Medtronic's Endeavor) promising greater efficacy and possibly increased safety. And the recent meta-analysis of DES and BMS in over a quarter of a million patients concluded:
In fact, David Kandzari now estimates that DES usage is back up -- now around 75% and will probably level off at 75-80%. But somehow we haven't been reading headlines about these reversals of fortune. I guess "About those tiny time bombs in your heart -- never mind" doesn't quite capture the headline writers' imaginations. Makes one pine for the good ol' days of 2006.... « permalink » « send comment » « back to top »
August 19, 2009 -- 5:45pm PDT FALSE: Stents Denied to Patients Over 59
Totally wrong! This lie has been circulated worldwide in an anonymous email and somehow has found its way into articles, such as the one mentioned, op-ed pieces, etc. and is clearly part of an organized campaign to scare elderly citizens into opposing health care reform. I am not going to get into the pros and cons of the overall plan here, but I do feel the need to publicize true facts over false rumors regarding stents and angioplasty.. The British National Health Service does not deny stents to patients over 59. This is an absurd claim, since it is specifically patients over 59 who are the prime beneficiaries of angioplasty, stents and interventional procedures. My sources are Dr. Peter Weissberg, medical director of the British Heart Foundation, as quoted in The Guardian, which states about the claim:
Also responding to this assertion was British Health Secretary Andy Burnham, who stated in an email to Dr. Hisham Rana's medical blog:
Stents and heart bypass surgery are fully available in the England, as they are and would continue to be in the U.S. It's possible that somehow, somewhere, someone picked up on a possible two-year-old hypothetical recommendation by the British National Institute for Health and Clinical Excellence (NICE) that drug-eluting stents might not be cost-effective. (We covered that topic in detail here -- and that recommendation was never adopted!) However, if you really want to discuss denial of health services, go to our Forum Topic titled, Financial Assistance for Plavix. Here you will read many stories from patients in the U.S. who received drug-eluting stents (most of them were insured for the procedure) but who were then denied reimbursement by their insurance companies for the recommended one-year-to-life prescription drug therapy of clopidogrel (Plavix) -- which is almost $1,500 annually. Many have stopped taking the drug because they cannot afford it. It is well-documented that premature cessation of antiplatelet therapy results in increased heart attacks and mortality. This is the true current status quo and to paraphrase the ophthamologist, "this situation in its present form might be lethal for you." « permalink » « send comment » « back to top »
July 29, 2009 -- 10:30pm PDT Is Radioactive Isotope Shortage an Opportunity
for CT Scans to Shine? Because of the two-part test and the various injections of technetium, etc., the test takes several hours to complete. The patient is also exposed to radiation. If the test is negative, the cardiologist assumes that there is no coronary artery disease (CAD), although there are definitely situations where false negatives can occur. If the blood flow to the patient's heart shows a deficit, then CAD is a prime suspect and the patient is usually sent to the cardiac catheterization lab for a diagnostic invasive angiogram and possible intervention (angioplasty or stent). The accuracy of the nuclear test is good, but 37% of patients sent for diagnostic angiograms show no disease -- indicative of a fairly high rate of false positives for the nuclear screening test, especially in women. Enter the Cardiac Computed Tomography Angiogram (CCTA).
Many in the imaging establishment have been using nuclear stress exams for many years, and the newer, more accurate CCTA has been fighting an uphill battle. But now, as reported in the New York Times, reactors in Canada and The Netherlands that produce technetium have shut down, for a while anyway, resulting in an emergency shortage of the isotope. The gravity of the situation is conveyed by Dr. Michael M. Graham, president of the Society of Nuclear Medicine -- “This is a huge hit,” he proclaimed to the Times. And Dr. Andrew J. Einstein of Columbia University College of Physicians and Surgeons pointed out that since this isotope is used to determine if a patient has a coronary blockage requiring an angioplasty or stent, those invasive procedures would be performed on some who did not need them. (He doesn't discuss the fact that this same isotope-based test sends many patients needlessly to the cath lab for an invasive angiogram -- a test that results in vascular complications about 3% of the time.) Nowhere in the article is the more accurate alternative test of CCTA mentioned. In fact, Dr. Einstein has been critical of CCTA in the past. Perhaps the shortage of technetium will drive more cardiologists to send patients for this newer test. Perhaps the diagnostic value of CCTA will be recognized as a result of the shortage of technetium. And it will shine...not glow... « permalink » « send comment » « back to top »
July 10, 2009 -- 1:50pm PDT Prasugrel / Effient Approved by FDA
Effient is manufactured by Eli Lilly and Company of Indianapolis, in partnership with Tokyo-based Daiichi Sankyo Ltd. Late Update: Here's part of the statement from the FDA:
« permalink » « send comment » « back to top »
July 5, 2009 -- 4:30pm PDT Stents: An Insider's Look
Currently two main companies are developing this technology: Volcano Corporation of San Diego, and LightLab, based in Massachusetts. To learn more about the growth of this imaging technology, read my exclusive interview with Dr. Guagliumi. |
 This
past month has offered an odd bit of reminiscence about an object
of ongoing interest: the drug-eluting stent (DES). First a study
from Duke was published in
This
past month has offered an odd bit of reminiscence about an object
of ongoing interest: the drug-eluting stent (DES). First a study
from Duke was published in  A
false meme has been circulating that the British National Health
Service (NHS) denies stents to patients older than 59...and that
Obama's health care reform will follow the NHS guidelines. I first
read this ridiculous assertion in a
A
false meme has been circulating that the British National Health
Service (NHS) denies stents to patients older than 59...and that
Obama's health care reform will follow the NHS guidelines. I first
read this ridiculous assertion in a  The
standard cardiac test for a symptomatic patient, one experiencing
chest pain, is usually a
The
standard cardiac test for a symptomatic patient, one experiencing
chest pain, is usually a  The
test is visual: a direct look at the coronary arteries. The test
does not involves exercise and stress, that in some patients is difficult.
The test takes less than 15 minutes to prep and complete. And with
modern equipment operated by technicians trained in the latest low-dose
protocols, the radiation exposure is less than that of a nuclear
test. And then there is the accuracy: 99% negative predictability
-- if the CCTA show no disease, you have no disease.
The
test is visual: a direct look at the coronary arteries. The test
does not involves exercise and stress, that in some patients is difficult.
The test takes less than 15 minutes to prep and complete. And with
modern equipment operated by technicians trained in the latest low-dose
protocols, the radiation exposure is less than that of a nuclear
test. And then there is the accuracy: 99% negative predictability
-- if the CCTA show no disease, you have no disease. After
a year-and-a-half of review, amid concerns over potentially serious
bleeding complications, the FDA today approved Eli Lilly's blood
thinner Prasugrel as a drug "to reduce the risk of blood clots
from forming in patients who undergo angioplasty" (it will
be marketed in the U.S. as Effient). Prasugrel will now be an alternative
to Bristol-Myers Squibb's Plavix, although the FDA approval comes
with a "black box warning" to physicians to be aware
of potentially fatal bleeding complications -- this warning would
ostensibly alert physicians to monitor patients carefully.
After
a year-and-a-half of review, amid concerns over potentially serious
bleeding complications, the FDA today approved Eli Lilly's blood
thinner Prasugrel as a drug "to reduce the risk of blood clots
from forming in patients who undergo angioplasty" (it will
be marketed in the U.S. as Effient). Prasugrel will now be an alternative
to Bristol-Myers Squibb's Plavix, although the FDA approval comes
with a "black box warning" to physicians to be aware
of potentially fatal bleeding complications -- this warning would
ostensibly alert physicians to monitor patients carefully.  It
remains to be seen what the availability of prasugrel will mean
for stent patients. Currently, patients receiving drug-eluting
stents are given Dual AntiPlatelet Therapy (DAPT) which is aspirin
for life and Plavix for a year or more. There have been a number
of studies regarding the optimum length for DAPT: some have shown
no benefit beyond six months, others indicate increased benefit
and protection against late stent thrombosis; one observational
study from the VA even noted that that there may be a "
It
remains to be seen what the availability of prasugrel will mean
for stent patients. Currently, patients receiving drug-eluting
stents are given Dual AntiPlatelet Therapy (DAPT) which is aspirin
for life and Plavix for a year or more. There have been a number
of studies regarding the optimum length for DAPT: some have shown
no benefit beyond six months, others indicate increased benefit
and protection against late stent thrombosis; one observational
study from the VA even noted that that there may be a " I
recently talked at length with
I
recently talked at length with 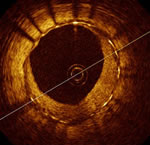 Dr.
Guagliumi reported results from the
Dr.
Guagliumi reported results from the