![]()
<< To Homepage >>
<<Archives>>
May-August 2010 Archives:
August 10, 2010 -- 6:15pm PDT DES 'n' DAPT: The OPTIMIZE Trial and Drug-Eluting
Stents Imagine their surprise. It's an issue that I testified about at the FDA drug-eluting stent safety hearings back in December 2006 -- and it's an issue that still has patients wondering what to do if they can't afford Plavix or need to have a surgical procedure less than a year after stenting. The purpose of those FDA hearings was to clarify safety issues with drug-eluting stents (DES) and to make recommendations as to the optimal duration of dual antiplatelet therapy (DAPT) necessary to reduce the risk of stent thrombosis (blood clotting inside the stent, often resulting in a heart attack). The upshot was that the recommended six months of DAPT was extended to twelve -- however, this was done in the absence of any real clinical data to back up the extension (no trials had been done to assess the optimal length of therapy). So it was a best-guess scenario for patient safety. That was four years ago. When I recently asked Dr. Eric Topol what we've learned since then, he replied: "Unfortunately, we don't know anything more regarding the appropriate length of dual antiplatelet therapy." So finally this year the DAPT trial began enrolling patients. DAPT will test whether there is any difference in outcomes between twelve months of DAPT and thirty. This multi-million dollar trial will not complete enrollment until 2013. And its results will only tell us if there is any difference between twelve and thirty months of DAPT. There will be no information about what happens if DAPT is less than a year -- information that might allow patients to stop taking this expensive drug therapy sooner. And there will be no breakdown in the type of drug-eluting stent -- somewhat unhelpful since several studies have shown that not all drug-eluting stents are alike. First generation DES like Cypher and Taxus will be lumped together with Xience and Endeavor. For example, if a specific drug-eluting stent has a faster healing rate (and in OCT imaging studies, Medtronic's Endeavor had healed more completely at six months than even bare metal stents) -- perhaps there's no need for a year or more of Plavix and aspirin -- at least with certain stents. Finally, there will be no genetic or platelet function testing, to see if certain patients with genetic variations are more prone to stent thrombosis than others. Why is this important information? Because stent thrombosis is an infrequent event. If it turns out that a specific subset of patients who are unresponsive to clopidogrel are also those who are experiencing the majority of stent thrombosis, then extended DAPT can be prescribed for just those patients...and the vast majority of patients do not need to take these drugs for such a long time, saving significant amounts of money, lessening bleeding complications, etc. Dr. Topol's take on the DAPT Trial: "I mean it's just crazy for me to think of the amount of resources that are being expended for mega trials like that. The notion that we should treat all patients for X duration is totally crazy. It completely goes against all the evidence that every patient is an individual with a separate biologic story.... I'm amazed that it's going forward." Enough said.
The results of these trials won't be known for a year or more. Meanwhile it is critically important for patients and physicians alike to be aware of the reality: the drug-eluting stent is a package deal -- it's both a stent and a year of antiplatelet drug therapy. Patients should be made aware of this prior to stent implantation to assure a high degree of compliance with the current guidelines -- and physicians should be sensitive to whether or not their patient will be able to comply, financially or clinically, before deciding on which stent to use. « permalink » « send comment » « back to top »
August 9, 2010 -- 10:14pm PDT The Un-Stent Wars: Call Out the Fractional
Flow Reserves But interest in FFR has taken off since the results of the FAME study were published back in January 2009. (FAME demonstrated that by measuring the differential in blood pressure across a blockage inside the coronary arteries, a more accurate assessment could be made as to whether or not to stent the narrowing -- more accurate when compared to angiography alone. The results: almost 1/3 less major cardiac events and 1/3 less stents implanted when FFR was used.) FFR's efficacy was enhanced further when last November the AHA/ACC/SCAI issued a Guidelines Update that increased the level of evidence and expanded the indications for FFR. So it would seem to be a no-brainer that during a coronary angiogram, a small wire could be threaded across a blockage and the need for a stent could be documented, relieving much of the anxiety and malpractice accusations of "too many unnecessary stents!" -- or the wire could show that a stent was not needed, saving the healthcare system a chunk of change. Another advantage of this technology for interventional cardiologists was being able to demonstrate that in complex 3 or 4 vessel disease, 1 or 2 of the narrowings were not causing ischemia, thus the patient could be done effectively using angioplasty and did not need to go through bypass surgery. At the time FAME was first presented at the 2009 TCT, only two companies manufactured FFR catheters: Volcano in San Diego (Nasdaq: VOLC) and Radi Systems in Sweden. Radi in fact had sponsored the FAME trial. But shortly after the TCT and just before publication of FAME in the New England Journal of Medicine, St. Jude Medical (NYSE: STJ) acquired Radi Systems. A captain of the device industry once told me that one reason larger companies buy smaller companies was to obtain their patents. And that's just how it played out a couple weeks ago when St. Jude Medical, a much larger device manufacturer than Radi or Volcano, filed a lawsuit against Volcano, involving five different patents used in St. Jude's (formerly Radi's) PressureWire® technology platform. Volcano fired back, stating that the "claims against it are entirely without merit, and looks forward to vindicating its rights in court." So the first "Un-Stent Wars" have begun: two companies battling as to who's technology can rightfully show that a stent is not necessary. One of Volcano's advantages is that their FloWire, ComboWire and PrimeWire products all work on an integrated platform with their IVUS and OCT intravascular imaging products, and can now be integrated into all major manufacturers' catheterization labs, saving time and money. St. Jude has been making similar advances, but lacks the IVUS technology. St. Jude, however, is a larger organization and it remains to be seen whether size matters, as CNBC commentator Herb Greenberg opines in his piece, "Can St. Jude Bigfoot Volcano?" As they say, "stay tuned".... « permalink » « send comment » « back to top »
July 21, 2010 -- 2:05pm PDT Transradial Aims At The SCAI Of course, in other parts of the world, such as Japan and India, there have been large scale live demonstration symposia for years, chaired, for example, by Dr. Shigeru Saito or Dr. Tejas Patel or others. In fact, many members of the core group of U.S. cardiologists practicing this approach got their initial training in these courses. Moreover, in other parts of the world, the transradial approach to PCI procedures is performed 40-50% of the time. Some cardiologists use the wrist access point 90% or more of the time. Transradial access has been in the news here lately, usually characterized as a "new kind of angioplasty" -- but it's not new, just new to the U.S. So having one of the major national professional heart societies like SCAI (and the one most targeted to interventional cardiology) sponsor a course just on the radial approach is a big deal. And why transradial? As you can read in the Transradial Center on Angioplasty.Org, it's safer with less complications, more comfortable for the patients, and a feature that will probably be the one to drive its acceptance most: it's more cost-effective. « permalink » « send comment » « back to top »
May 27, 2010 -- 3:50pm EDT Stent Helpers: IVUS, OCT and FFR -- Intravascular
Tools At EuroPCR Briefly, OCT and IVUS are ways of imaging the inside of a coronary artery during a standard invasive catheterization or angioplasty. Both of these imaging technologies can help the cardiologist see things not visible on standard X-ray angiography (an angiogram is essentially a "shadow" image). The placement, sizing and expansion of a stent can all be measured more accurately via intravascular imaging. There is a difference between OCT and IVUS. OCT is a far higher resolution image, but IVUS penetrates the arterial wall deeper, revealing anatomical information hidden below the surface. All of this is discussed in our interview with OCT expert Dr. Giulio Guagliumi who discusses how both imaging modalities are useful and that the "combination is the true projection for the future". FFR measures intracoronary blood pressure and has been shown in the FAME study to be useful in decision-making about whether or not a blockage needs to be stented. In FAME, FFR-guided stenting used 1/3 less stents and improved outcomes by 28%. Recent news for OCT was that earlier this month the FDA approved LightLab's OCT device -- the first approval for this technology in the United States. Two weeks later, having seen a future for OCT, St. Jude Medical announced it was acquiring LightLab for $90 million. A competing OCT system from Volcano currently has European approval and is awaiting an okay from FDA; the company anticipates commercial release in the U.S. sometime mid-2011. Volcano has been a leader in IVUS imaging since its inception and at EuroPCR announced a new digital IVUS catheter, the lower profile Eagle Eye® Platinum, which is more deliverable and can fit through a 5F guide, making it ideal for transradial procedures done via the wrist. The only other IVUS system is the iLab, manufactured by Boston Scientific. An interesting new cross-over product was also introduced by Volcano, the VIBE™ RX Vascular Imaging Balloon Catheter, which combines IVUS with a dilatation balloon to provide image-guided therapy. This is a concept being explored by Volcano -- and a future system of Forward-Looking IVUS may make inroads into solving the problem of chronic total occlusions (CTO). Andreas Gruentzig, the inventor of coronary angioplasty, once told me that unless angioplasty could solve the problem of CTOs, it would never be able to completely compete with bypass surgery. Finally FFR was in the news as the two-year FAME data, first presented at TCT last September, were published online in JACC. The original findings were sustained, leading to the conclusion that:
This conclusion is one reason why 18 months ago St. Jude Medical (them again?) acquired the Swedish manufacturer of FFR catheters, Radi Medical Systems. The competing FFR system is made by...Volcano (them again?) and it just received FDA and CE Mark approval for its latest FFR product, the PrimeWire PRESTIGE™ Pressure Guide Wire. So, to recap the acquisitions and new product introductions: St. Jude Medical now offers FDA-approved OCT and FFR; Boston Scientific offers IVUS; Volcano offers IVUS, FFR and anticipates FDA approval of OCT -- the company is also poised to launch various new "image-guided therapies". (BTW, a report yesterday on theheart.org states that, with its recent acquisitions, "St Jude boasts the only intravascular ultrasound platform with both OCT and fractional-flow-reserve technology." To our knowledge, St. Jude does not offer an IVUS system, unless they bought Boston Scientific when we weren't looking.) One last point is that the advantage of utilizing products from the same manufacturer is being able to use the same console or the same integrated cath lab system with all devices, especially if they are used in combination, in order to minimize the extra time and effort required to add on these intravascular "stent helpers" for the benefit of patients and procedural outcomes. « permalink » « send comment » « back to top »
May 16, 2010 -- 2:30pm EDT Stent Inventor's New Ideas Dr. Palmaz developed his revolutionary balloon-expandable coronary stent while a professor at the University of Texas Health Science Center (UTHSC) in San Antonio back in the late '80s, along with Dr. Richard Schatz, and the university has since made millions in royalties from his invention. In the article, Steve Solomon, CEO of Palmaz Scientific, states that his company "will use a portion of the money to help secure approval in the European market of its Sesame stent, which has a special microscopic mesh coating that helps reduce the effect of impurities in the metal tube. The state funds also will be devoted to buying equipment and hiring engineers to spur the development of next-generation stents."
His company's technology utilizes nanotechnology and physical vapor deposition (PVD) to yield material of higher purity, lower profile and a micro-grooved flow surface so that the stent can have an all metal micromesh covering only 5 microns thick (about the size of a blood cell) with openings less than 100 microns. The concept is that this covering can act as a screen to prevent migration of the stent struts into the plaque, which can cause inflammation and embolization of the plaque, and that it can promote healing, resulting in reduced restenosis without having to subject the patient to long-term dual antiplatelet therapy or the risk of late stent thrombosis. These ideas are, of course, controversial -- especially in the current world of drug-eluting polymers and bioabsorbable stents -- but controversy is nothing new for Dr. Palmaz. More information about this technology can be found at Palmaz Scientific's web site. « permalink » « send comment » « back to top »
May 4, 2010 -- 3:37pm EDT Where Can I Get a Stent or Angioplasty
Done Through the Wrist? But in the United States, the wrist approach is utilized in less than 5% of cases. The reason is public education and professional training: patients don't know about it; many cardiologists haven't learned how to do it. Cardiologists are trained in fellowship programs and since most fellowship programs don't have "radialists" on the team, new cardiologists never learn the technique. But this is changing. There's been an uptick in interest certainly, but also in numbers. Still the U.S. lags behind. Which is why Angioplasty.Org offers two important pages: one, for cardiologists and staff, is a list of training opportunities -- usually one or two day courses to learn about the wrist technique and to even get some "hands on" experience (pun intended). But patients, who are about to have an angiogram or angioplasty or stent placement, should look over our Radial Hospital Locator -- a listing of scores of hospitals and practitioners who offer the radial approach. (And if you are a transradial cardiologist and are not listed, add your hospital or practice here.) Why should patients want to have their procedure done via the wrist? A few benefits are:
As Dr. Howard Cohen of Lenox Hill Hospital in New York said to us about the wrist technique:
« permalink » « send comment » « back to top »
May 2, 2010 -- 6:45pm EDT Stents and Angioplasty from the Wrist:
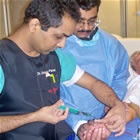
Texas Style When Dr. Patel arrived in Houston, radial procedures were not well-known. In fact, as he told me:
Not only were the physicians and staff unfamiliar with doing these complicated interventions from the wrist artery -- the patients were as well:
"Well-known cardiologists around the world" is correct -- in other countries, up to 50% of all catheter-based coronary procedures are done from the transradial wrist approach. The reason the U.S. is so far behind (only 5% of procedures are done this way here) has to do with training. Cardiologists learn the femoral approach in their fellowships and, unless they fortuitously land a position in a teaching program like Dr. Coppola's, they are unfamiliar with the radial approach. To learn it, they have to go back to the drawing board, attend a specialized training course, taking them out of their practice for a short while, and they have to do the procedure often enough to overcome the learning curve. Of course, once they do learn, they become sought after, as Dr. Patel has in the hospitals where he practices. His first radial procedure is shown in the photo above (note the smiling patient!) and a story in the local paper about it made a big difference. Suddenly patients were interested and referring physicians were sending him their patients. That was two years ago; he has now done over a thousand radial procedures. As he told me:
There's been such interest that Dr. Patel is now running monthly transradial training courses for other cardiologists. As more cardiologists learn the technique and more patients find out that they may not have to lie flat on their backs for hours, use of the transradial approach in the U.S. is sure to make substantial increases. You can read my complete interview with Sanjay Patel, MD, FACC in Angioplasty.Org's Transradial Center. |
 At
the
At
the  Enter
Enter  FFR
(Fractional Flow Reserve) is a technology I've written about
many times. You can read about it in depth in our article, "
FFR
(Fractional Flow Reserve) is a technology I've written about
many times. You can read about it in depth in our article, " The
Society for Cardiovascular Angiography and Interventions (a.k.a.
SCAI) has just stepped up to the plate -- the training plate, that
is, and it's just a few blocks from Fenway Park in Boston. On Friday,
November 5, 2010, at the Sheraton Boston Hotel, SCAI will present
what I believe is the
The
Society for Cardiovascular Angiography and Interventions (a.k.a.
SCAI) has just stepped up to the plate -- the training plate, that
is, and it's just a few blocks from Fenway Park in Boston. On Friday,
November 5, 2010, at the Sheraton Boston Hotel, SCAI will present
what I believe is the 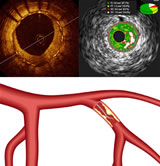 This
graphic shows three intravascular imaging/therapeutic modalities
that were making news this month, both in the U.S. and in Paris
at the EuroPCR meeting ending tomorrow. Clockwise they are
Optical Coherence Tomography (OCT), Intravascular Ultrasound
(IVUS) and Fractional Flow Reserve (FFR). You can read more
about these in depth in Angioplasty.Org's
This
graphic shows three intravascular imaging/therapeutic modalities
that were making news this month, both in the U.S. and in Paris
at the EuroPCR meeting ending tomorrow. Clockwise they are
Optical Coherence Tomography (OCT), Intravascular Ultrasound
(IVUS) and Fractional Flow Reserve (FFR). You can read more
about these in depth in Angioplasty.Org's  Stent
pioneer Dr. Julio Palmaz was in the news this week regarding
a $3 million investment made in his company
Stent
pioneer Dr. Julio Palmaz was in the news this week regarding
a $3 million investment made in his company 
 At
At  Two
years ago interventional cardiologist Dr. Sanjaykumar Patel
joined the Sadler Clinic in The Woodlands, a suburb of Houston.
He had taken his Fellowship at St. Vincent's Hospital in New
York, with Dr. John Coppola as his mentor. Dr. Coppola is a
strong advocate of the transradial approach, in which angioplasty
and stents are delivered to the coronary arteries via a small
puncture in the wrist. (Most of these procedures in the United
States are done through the femoral artery in the groin.) You
can
Two
years ago interventional cardiologist Dr. Sanjaykumar Patel
joined the Sadler Clinic in The Woodlands, a suburb of Houston.
He had taken his Fellowship at St. Vincent's Hospital in New
York, with Dr. John Coppola as his mentor. Dr. Coppola is a
strong advocate of the transradial approach, in which angioplasty
and stents are delivered to the coronary arteries via a small
puncture in the wrist. (Most of these procedures in the United
States are done through the femoral artery in the groin.) You
can