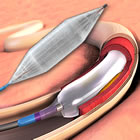
Lutonix and IN.PACT drug-coated balloons
Last week saw the U.S. Centers for Medicare and Medicaid Services (CMS) approve reimbursement for the two drug-coated balloons that recently were approved by the FDA: C. R. Bard’s Lutonix and Medtronic’s IN.PACT.
C. R. Bard’s Lutonix drug-coated balloon (DCB) was approved in October 2014, while Medtronic’s IN.PACT Admiral was approved in January of this year. Both devices have shown superior results when compared to uncoated balloons (a.k.a. “plain old balloon angioplasty” or POBA). Continue reading

 What does a Denial of Service Attack have to do with stents, angioplasty and PCI?
What does a Denial of Service Attack have to do with stents, angioplasty and PCI?
 Published “online first” today in the New England Journal of Medicine are two articles, authored by the Democratic and Republican presidential nominees, President Barack Obama and former Massachusetts Governor Mitt Romney, describing their health care platforms and their visions for the future of American health care. The editors of NEJM had asked the nominees for these statements which are brief and concise, summing up the two positions in less than 1,300 words each.
Published “online first” today in the New England Journal of Medicine are two articles, authored by the Democratic and Republican presidential nominees, President Barack Obama and former Massachusetts Governor Mitt Romney, describing their health care platforms and their visions for the future of American health care. The editors of NEJM had asked the nominees for these statements which are brief and concise, summing up the two positions in less than 1,300 words each. The question of the day, regarding whether or not to stent a coronary artery, is now being brought to the forefront by the U.S. government in the form of a
The question of the day, regarding whether or not to stent a coronary artery, is now being brought to the forefront by the U.S. government in the form of a 

