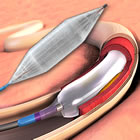
Lutonix and IN.PACT drug-coated balloons
Last week saw the U.S. Centers for Medicare and Medicaid Services (CMS) approve reimbursement for the two drug-coated balloons that recently were approved by the FDA: C. R. Bard’s Lutonix and Medtronic’s IN.PACT.
C. R. Bard’s Lutonix drug-coated balloon (DCB) was approved in October 2014, while Medtronic’s IN.PACT Admiral was approved in January of this year. Both devices have shown superior results when compared to uncoated balloons (a.k.a. “plain old balloon angioplasty” or POBA). Continue reading



 Bristol-Myers Squibb (NYSE: BMY) is no longer offering its $37-a-month Plavix® Co-Pay Discount Card. And some patients are not happy about this. Additionally, according to the company’s web site for “
Bristol-Myers Squibb (NYSE: BMY) is no longer offering its $37-a-month Plavix® Co-Pay Discount Card. And some patients are not happy about this. Additionally, according to the company’s web site for “ Today the editors of the HEART Group Journals, comprising the Journal of the American College of Cardiology and other participating cardiovascular publications, issued a “
Today the editors of the HEART Group Journals, comprising the Journal of the American College of Cardiology and other participating cardiovascular publications, issued a “


