December 7, 2006
Cardium's
Generx Advances to Phase 3 Following Meeting with FDA
(source: Cardium Therapeutics, Inc.)
The company announced that Generx™ (alferminogene tadenovec) is to be advanced
to a Phase 3 clinical trial in women as a potential treatment for myocardial
ischemia (insufficient blood flow within the heart muscle), following a meeting
with the U.S. Food and Drug Administration (FDA). Generx represents a new therapeutic
class of biologics designed to promote angiogenesis, a natural process of blood
vessel growth within the heart muscle, following a one-time intracoronary administration
from a standard cardiac infusion catheter. “Generx is the first and only DNA-based
cardiovascular therapeutic to be advanced to Phase 3, and is believed to be the
only current Phase 3 product candidate for the potential treatment of stable
angina, a chronic medical condition affecting millions of patients in the U.S.
and elsewhere,” stated Christopher J. Reinhard, Cardium’s Chairman and Chief
Executive Officer.
November 30, 2006
Cardium
Announces Formation of Scientific Advisory Board
(source: Cardium Therapeutics, Inc.)
Cardium Therapeutics, Inc. today announced the formation of a 6-member Scientific
Advisory Board (SAB). The SAB, which includes physicians, clinicians, and biotechnology
industry professionals, will provide counsel on a broad range of scientific
and product development issues." We are extremely pleased to have this distinguished
group of leading scientists and physicians join Cardium's inaugural Scientific
Advisory Board," stated Christopher J. Reinhard, Cardium's Chairman and Chief
Executive Officer. "Their deep knowledge and experience will be important as
we look to advance our product candidates currently in development toward commercialization."
November 30, 2006
NYS
Report Ranks LIJ, North Shore University Hospital Among New
York's Best for Angioplasty Survival (source: North
Shore - Long Island Jewish Health System)
“Long Islanders should be thankful to know that of the five hospitals in their
region reviewed in the state Health Department’s most recent report, three had
outcomes that were the best in the state,” said Dennis Dowling executive director
of NSUH and LIJ, noting that Winthrop University Hospital in Mineola was the
only other hospital in the state besides North Shore and LIJ to achieve the double-star
designation. “In this era of public disclosure of clinical outcomes, patients
and their families should take the opportunity to avail themselves of this highly
useful data compiled by the state.”
November 13, 2006
American
College of Cardiology and Partners Launch National Alliance
To Reduce Door-to-Balloon (D2B) Times
The American College of Cardiology (ACC), together with the American Heart
Association (AHA) and other key national healthcare organizations, announces
the launch of its latest quality campaign, “Door to Balloon (D2B): An Alliance
for Quality”, aimed at improving the timeliness of lifesaving therapy for patients
with heart attacks at the nation’s hospitals that perform emergency angioplasty. (Read
more...)
October 24, 2006
Cardium
Reports on Positive Effects of Hypothermia Following Heart
Attack and Announces Clinical Study to Be Co-Sponsored by
Swedish Cardiology Center
(source: Cardium Therapeutics, Inc.)
Cardium Therapeutics and its subsidiary InnerCool Therapies today reported
on preclinical data demonstrating a new and expanded benefit of early rapid
cooling for the potential treatment of acute myocardial infarction (heart attack),
as presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2006 Annual
Meeting in Washington, DC. The data showed that cooling prior to PCI reduced
overall infarct size (reflecting tissue damage) by an additional 40%. These
findings strongly support the use of early rapid cooling in planned clinical
studies, and suggest that InnerCool's endovascular cooling system may have
the potential to enable interventional cardiologists to dramatically reduce
tissue damage following a heart attack.
October
16, 2006
Paragon
Intellectual Properties Announces Funding Commitments
of $15 Million
(source:
Paragon
Intellectual
Properties,
LLC)
A new West Virginia start-up device company is opening an R&D center in
southern California to develop new stents, nanotechnology, and other innovations
-- funded by a $15 million in financing from private investors and equity stakes
made by both Abbott and Boston Scientific. (Read
more...)
October
12, 2006
David
Kandzari, M.D., International Expert in Interventional
Cardiology, Named Chief Medical Officer of Cordis Cardiology
(source:
Cordis Corporation)
Cordis has announced that effective December, Dr. Kandzari will oversee medical
and clinical affairs for the company. Dr. Kandzari becomes the fourth high
profile interventional cardiologist to join a medical device firm in the past
year.
October 2, 2006
Bypass
Surgery Tops Angioplasty for Sickest Heart Patients
(source: Duke University Medical Center)
Bolstering some studies that have been discussed for years, Duke's analysis
shows that the sickest patients, mainly those with diffuse, multivessel coronary
artery disease may best be served by Coronary Artery Bypass Grafting (CABG)
rather than angioplasty. Survival rates were 5.3 months longer in the surgically-treated
patients, as opposed to those who had angioplasty and stents. A very important
caveat to this study is that the data predated the introduction of drug-eluting
stents, which have been shown to significantly reduce restenosis and reclogging.
Whether these will show a prolonging of life and change the surgery vs. angioplasty
debate can only be verified by future studies. Another concern is whether the
recent reports about the incidence of late stent thrombosis in drug eluting
stents will significantly affect patient outcomes.
September 26, 2006
FDA
Approves Cordis Carotid Stenting System
(source: Cordis Corporation)
Cordis announced that the FDA has approved its PRECISE® Nitinol Stent and ANGIOGUARD® Emboli
Capture Guidewire system for use in opening up and stenting carotid arteries.
This device now joins the two other carotid stent systems on the market in
the U.S. -- Abbott's Xact/Emboshield system and the ACCULINK/ACCUNET system
that was created by Guidant, but is now also owned by Abbott.
September 23, 2006
Report
States FDA Drug Safety System is Broken
In a sweeping critique of the way in which the U.S. Food and Drug Administration
(US FDA) currently monitors the safety of drugs, a blue ribbon panel of the
Institute of Medicine (IOM), part of the National Academies, has issued a 350
page report that calls for greatly increased funding and action from Capitol
Hill. (Read
more...)
September 23, 2006
"I
drove home and reached for three of the most useful medicines
I know: aspirin, acetaminophen (Tylenol) and the Internet"
Commentary on an Op-Ed piece in the New York Times by Jerry Avorn of Harvard
Medical School about the dissemination of useful medical information. (Read
more...)
September 21, 2006
Dr.
Michael Morgan Reports on Benefits of InnerCool's Celsius
Control System in Inducing Hypothermia During Aneurysm Surgery
(source:
Cardium Therapeutics, Inc.)
At a meeting of the Neurosurgical Society of Australasia, the experience in
26 patients with cooling the brain via catheter during aneurysm surgery in
order to limit damage was reported on -- the outcomes being very positive.
September 19, 2006
Benefits
of Vasomedical's EECP Therapy in Heart Failure Presented
at the 10th Annual HFSA Scientific Meeting
(source: Vasomedical, Inc.)
Data was presented by physicians from New Delhi, India to St. Peterburg, Florida
and Stony Brook, New York to show improvements in heart failure patients in
a number of areas, among them exercise capacity, quality of life and angina.
September 12, 2006
Angioplasty.Org
Cited as Model in Australian Government Campaign to Improve
Physician-Patient Communication
The Australian National Health and Medical Research Council has used Angioplasty.Org
as a resource and case study, in a newly published 163-page healthcare toolkit
intended to improve practices in physician-patient communication. "Angioplasty.Org
provides information and tools to help patients make informed decisions about
heart disease treatment," explains Deborah Shaw, Angioplasty.Org Education
Editor. "It's great validation to see content we publish recognized by the
NHMRC and recommended to healthcare professionals and consumers throughout
Australia.
(Read
more...)
August 31, 2006
U.S.
Court Grants Injunction Against Generic Plavix
A U.S. District Court today granted Sanofi/Bristol-Myers' request for a preliminary
injunction against Apotex, prohibiting the Canadian-based firm from selling
their generic clopidogrel product while the dispute over the validity of Sanofi's
branded Plavix is decided. (Read
more...)
August 30, 2006
Abbott
Announces Completion of Enrollment in Groundbreaking Vulnerable
Plaque Study
Utilizing the Volcano Therapeutics' intravascular ultrasound imaging system,
the PROSPECT study, headed by Dr. Gregg Stone of Columbia University Medical
Center in New York, will attempt to more clearly define who is at risk for
a heart attack by looking at vulnerable plaque, not just plaque "build-up". (Read
more...)
August 29, 2006
Positive
PEECH Trial Results and Related Article Highlighting
Mechanism of Action Published in the Journal of the American
College of Cardiology
(source: Vasomedical, Inc.)
Enhanced External Counterpulsation (EECP) is a noninvasive, outpatient therapy
for the treatment of diseases of the cardiovascular system currently indicated
for use in cases of stable or unstable angina, congestive heart failure, acute
myocardial infarction and cardiogenic shock. The upcoming issue of the Journal
of the American College of Cardiology features an article (abstract below)
detailing the positive benefits of this therapy for patients with refractory
angina. The article concludes:
EECP treatment reduces arterial stiffness
and improves wave reflection characteristics in patients
with refractory angina. These changes decrease LV afterload
and myocardial oxygen demand and reduce the number of angina
episodes, therefore enabling patients to participate in
continuous exercise programs which in turn may provide
long-term benefits and sustained improved quality of life.
The JACC article did not set out to determine
the long-term effects of the therapy, but a number of patients
have posted their anecdotal experiences with EECP in Angioplasty.Org's
Discussion Forum (link below)
related stories:
Enhanced
External Counterpulsation
Treatment Improves
Arterial Wall Properties
and Wave Reflection
Characteristics in
Patients With Refractory
Angina -- Journal
of the American College
of Cardiology
Experiences
with Enhanced External
Counterpulsation
(EECP) -- Discussion Forum
-- Angioplasty.Org
August 28, 2006
Gerald
Ford discharged after successful angioplasty
Former President Gerald Ford, age 93, underwent successful angioplasty on Friday,
including placement of two stents. The procedure was done at the Mayo Clinic
in Rochester, Minnesota. (Read
more...)
August 22, 2006
New
York Times: "In an Ohio City, Heart Procedure is Off
the Charts"
A regional hospital in the small city of Elyria, Ohio got some publicity last
Friday morning that they may have preferred not to. The EMH Regional Medical
Center was the subject of a front page story in the New York Times on August
18. At the center of the story is the very high rate of angioplasty procedures
performed on Medicaid patients at this hospital: almost four times the national
average. The article notes that the outcomes for the hospital are very good
-- its heart program has been highly rated -- but there are many questions
raised by the article, and by the study on which it was based. (Read
more...)
August
15, 2006
Patient
Power: Making Sure Your Doctor Really Hears You
(source:
Deborah Franklin,
New York Times)
Very good article for patients (and physicians) in the New York Times Health
section, discussing the "communications gap" between doctors and
their patients, and some suggestions for improvement. The article surveys opinions
from those in a new book titled, “You: The Smart Patient: An Insider’s Handbook
for Getting the Best Treatment,” by doctors Michael F. Roizen and Mehmet C.
Oz to medical sociologist Richard Frankel of Indiana University, who worked
on a physician training program now used by Kaiser Permanente. The piece also
discusses the role of the Internet in this relationship, quoting Dr. Carma
Bylund of Sloan-Kettering as saying "Some patients now come armed with
sheaves of journal articles and printouts, and demands for specific treatment." Angioplasty.Org
hears the same statement from virtually every cardiologist queried.
related stories:
You
and Your Physician -- Angioplasty.Org
Patient Center
August
9, 2006
Marvin
L. Woodall Named CEO of Cardiovascular Research Foundation
(source:
Cardiovascular
Research
Foundation)
Marvin L. Woodall, a long-standing member of the Board of Directors of the
Cardiovascular Research Foundation (CRF), was named the Chief Executive Officer
(CEO) of the organization effective immediately. The organizations's press
release states that "The position of CEO is newly created in an effort
to both manage the tremendous growth experienced by CRF over the past several
years as well as to lead the Foundation in new endeavors in the future." The
CRF produces the annual
TCT meeting each fall in Washington DC (this year from October 22-27).
August 8, 2006
Generic
Version of Plavix® (Clopidogrel) is Launched by Apotex
In a bold and risky move, Apotex has launched a generic version of the top-selling
antiplatelet drug Plavix. The generic drug, clopidogrel bisulfate, was approved
by the U.S. Food and Drug Administration in January. Plavix is a blood thinner,
taken by millions of heart patients and prescribed for all drug-eluting stent
patients to lower the possibility of "stent thrombosis", a serious and sometimes
fatal clotting of blood inside of the stent. (Read
more...)
July 28, 2006
Will
Multislice CT Angiography Replace Catheterization?
A study from the Cleveland Clinic was published earlier this week in JAMA comparing
multidetector computed tomography ((a.k.a. Multislice CT) to the more invasive
catheter-based angiography, currently considered the "gold standard" for measuring
the blockage in coronary arteries. News media reports on the study were mixed.
So one might wonder exactly what the study means for patients: are CT coronary
scans good or not? (Read
more...)
July 25, 2006
SHAPE
Guidelines On Diagnosis Generate Heated Controversy
These recommendations were characterized in one medical publication as "a bold
new report", yet in another they were labeled as "scientifically extremely
questionable" and they "should be repudiated". In the public realm, today's
Boston Globe ran a feature story questioning the apparent conflict of interest
involved by who paid for the publication of the guidelines, which ran as a
supplement in the July 17 issue of the American Journal of Cardiology. (Read
more...)
July
21, 2006
Hospitals
snap up 64-slice heart scanners
(source:
Alan Bavley,
The News-Sentinel,
Fort Wayne,
Indiana)
This feature article covers a wide range of topics concerning the rapidly growing
use of 64-slice CT scans to diagnose coronary artery disease, a subject not
without controversy. The article states an oft-quoted fact, that one-third
of all diagnostic cardiac catheterizations (angiograms) turn out to be normal.
Most current studies of Multislice CT scans show a negative predictive value
in the high 90's (the patient does not have significant disease) -- at a cost
that 10% of a standard angiogram and non-invasive.
related stories:
Multislice
CT Angiogram -- Angioplasty.Org
Interview
with Daniel S.
Berman, MD -- Angioplasty.Org
July 10, 2006
New
guideline for screening apparently healthy individuals to
prevent a heart attack
(source:
Association
for Eradication
of Heart
Attack)
A group of influential cardiologists, imaging and other specialists has produced
a dynamic and somewhat controversial report, which appeared as a supplement
to today's American Journal of Cardiology. In it, the authors call
for carotid ultrasound and CT scanning for all "at risk" men and
women, defined as those men over 45 and women over 55 who meet one or more
of the following risk factors, even if they have no symptoms: raised cholesterol
(> 200mg/dL), high blood pressure (> 120/80 mm Hg), diabetes, family
history of heart disease, metabolic syndrome or smoking. The task force named
themselves SHAPE (Screening for Heart Attack Prevention and Education). (Read
more...)
June 27, 2006
Congressman's
Inquiry Finds F.D.A. Enforcement Down 54% Under Bush Administration
The senior Democrat on the House Government Reform Committee, Congressman Henry
A. Waxman of California, issued the results of his 15-month investigation into
enforcement actions undertaken by the U.S. Food and Drug Administration (FDA)
and found that last year the Agency issued 535 warning letters to medical device
or pharmaceutical companies -- this was compared to the year 2000, when 1,154
letters were issued -- a 54 percent decline and the lowest number in 15 years
-- Waxman terms this "a precipitous drop in FDA enforcement actions". (Read
more...)
June 26, 2006
Boston
Scientific Recalls Some Pacemakers and Defibrillators
Boston Scientific announced that it is "retrieving" from hospital inventories
and its sales force a specific subset of units that span various models of
heart rhythm devices. The problem, which the company says is low in frequency,
has to do with a low-voltage capacitor that has been performing below expectations
and may cause a variety of device failures or premature battery depletion. (Read
more...)
June 8, 2006
New
Stent Delivery System Allows Physicians to Treat Life-Threatening
Biliary Blockages
(source:
Cordis
Corporation)
Cordis today launched the PALMAZ® BLUE™ .014 Transhepatic Biliary Stent --
this is their latest biliary stent, a device which is often used to open blockages
in the bile ducts to promote the flow of fluids from the liver, gallbladder
and pancreas to the small intestines. Their press release states that each
year, more than 30,000 Americans are affected by life-threatening blockages
in the bile ducts, leading to the need for treatment.
June
8, 2006
'Balloon
op' could cut deaths by a third
(source:
Claire
Lomax,
This
is
Bradford,
UK)
A news feature from a northern England paper discusses how angioplasty can
stop a heart attack and save lives. Bradford Royal Infirmary patients are now
taken by ambulance to Leeds General Infirmary where they will be fast-tracked
to undergo immediate emergency angioplasty.
related stories:
Heart
Attack
and Angioplasty -- Angioplasty.Org
June
7, 2006
Papers
Show Guidant Considered Warning Doctors of Hazards But
Didn't Send Letter
(source:
Barry
Meier,
New
York
Times)
An article in today's New York Times by Barry Meier, the reporter who "broke
the news" about defects in Guidant defibrillator and CRT units last year,
reports that papers released to the Times by a Texas judge show that Guidant
had prepared a "Dear Doctor" letter in January 2005 explaining defects
in its Contak Renewal devices, but apparently decided against sending it. An
inquiry by the US FDA and Justice Department has been ongoing and the NYT article
states that this evidence suggests "the legal and financial consequences
from that inquiry could be significant for Guidant and Boston Scientific, which
completed its acquisition of Guidant in April." Boston Scientific wouldn't
comment on the specifics, but did state, "We understand and acknowledge the
need for more timely and transparent communication. We are committed to doing
a better job of communication with patients and physicians." The letter is
one of 22 documents requested by the New York Times and are among 600,000 pages
that had been submitted to the Texas court as part of a product liability case.
The Times has been seeking these documents and it was only after an emergency
appeal by Guidant attorneys was rejected that the documents were released.
The judge has given Guidant until the close of business today to show why the
rest cannot be released as well.
related stories:
Ruling
Could
Put Guidant
Documents
On Display -- Wall
Street
Journal
$$
Boston
Scientific
unit
faces
suit -- Bloomberg
News,
via the
Boston
Globe
Guidant
Letter
About
Risks
Never
Sent -- Mark
Jewell,
Associated
Press
June
6, 2006
Effect
of Door-to-Balloon Time on Mortality in Patients With
ST-Segment Elevation Myocardial Infarction
(source: McNamara, Krumholz
et al, Journal of the American College of Cardiology)
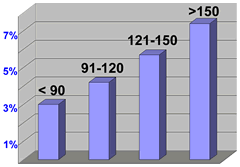
In-Hospital
Mortality vs. Door-to-Balloon Time
|
|
Something many
people still don't realize is that opening up a blocked
coronary artery in the midst of a myocardial infarction
(a.k.a. heart attack) can actually stop the heart attack
and significantly reduce damage to the heart muscle. The
trick is to get the patient to the cath lab quickly enough.
The ACC/AHA Guidelines recommend a "door-to-balloon
time" of 90 minutes or less, but as this report states,
only a minority of patients are treated within these guidelines
-- and most importantly, this statistic has not changed
recently. This report, which looked at over 29,000 patients,
recommends a concerted effort across the various entities
involved, the Emergency Medical Services (EMS) that bring
the patient to the hospital, the Emergency Room staff,
the entire internal scheduling flow system within the hospital
and more. |
related stories:
Speeding
up primary PCI saves lives -- theheart.org
Heart
Attack and Angioplasty -- Angioplasty.Org
June
6, 2006
Boston
Scientific Launches New Balloon Catheters Designed to
Address Wide Variety of Peripheral Intervention Needs
(source:
Boston
Scientific
Corporation)
These two Sterling™ balloons in monorail and over-the-wire configurations
were actually cleared for marketing by the FDA back in December 2005 and are
just now being launched. The FDA 510(k) clearance stated that these new balloons
are "considered to be substantially equivalent to the Ultra-Soft SV Balloon
Dilatation Catheter" that Boston Scientific has marketed since 2002. In
January 2006 the company received a
warning letter from the FDA that effectively put on hold any future approvals
for Class III devices (the Sterling balloons are Class II and, as stated, were
approved prior to the warning letter).
related stories:
510(k)
Summary
for
Sterling™ Over-The-Wire
Balloon
Catheter -- Food
and
Drug
Administration
(PDF)
510(k)
Summary
for
Sterling™ Monorail
Balloon
Catheter -- Food
and
Drug
Administration
(PDF)
June
5, 2006
Cardium
Reports Meta-Analysis of Angiogenic Gene Therapy Studies
Shows Positive Effects in Men and Women With Heart Disease
(source:
Cardium
Therapeutics,
Inc.)
Dr. Timothy D. Henry, an interventional cardiologist and Professor of Medicine
at the Minneapolis Heart Institute, presented an analysis of the AGENT clinical
data on Saturday at the 9th Annual Meeting of the American Society of Gene
Therapy (ASGT). The data, which relates to Cardium's lead product candidate,
Generx™ (Ad5FGF-4) showed positive results in both men and women treated. Anginal
pain was reduced significantly in patients vs. placebo. Previously reported
reductions in symptoms at 6 months were maintained at one year. Additionally
exercise capacity on a treadmill test increased for those patients over 55
who previously could not exercise for more than 5 minutes. The exercise capacity
continued to increase between 6 months and one year, even though the therapy
consists of a one-time infusion of the angiogenetic agent. Cardium is planning
to launch further clinical trials later this year. Stay tuned -- Angioplasty.Org
will soon be launching a special section on the exciting new frontier of angiogenesis
in the treatment of heart disease.
June
5, 2006
Heart
attack, bypass patient less fit than previously thought
(source: American Heart Association)
That goes for stent and angioplasty patients as well! The importance of cardiac
rehabiliation in the recovery of patients is stressed in this report, as stated
by Philip A. Ades, M.D., lead author and professor of medicine and director
of Preventive Cardiology and Cardiac Rehabilitation at the University of Vermont
College of Medicine in Burlington. “The biggest surprise was how low the fitness
levels were in women. The average woman in this study fell in the fitness range
where cardiologists often consider heart transplantation in heart failure patients.
The take-home message to cardiac surgeons and interventional cardiologists
is that the job is only half-done when bypass surgery or coronary stenting
is satisfactorily performed. These patients remain sorely in need of rehabilitation
despite optimal in-hospital care.”
June
5, 2006
FoxHollow
Technologies Names Dr. John Simpson Chief Executive Officer
(source:
FoxHollow
Technologies,
Inc.)
Angioplasty pioneer Dr. John Simpson, who founded FoxHollow, has now been named
CEO. Simpson had been serving as interim CEO since the retirement of Robert
W. Thomas in January.
June 2,
2006
Medtronic
Announces Key Milestones in Endovascular Repair at Society
for Vascular Surgery Meeting
(source: Medtronic, Inc.)
At the 60th annual Society for Vascular Surgery meeting Medtronic celebrated
several milestones in its company history of pioneering endovascular device
manufacturing, among them over 10 years and 100,000 endografts placed (for
abdominal aortic aneurysms or thoracic lesions). The importance of these devices
was summed up by Katie Szyman, VP and general manager of Medtronic's Endovascular
business: "The advent of stent graft therapy has given patients an effective,
minimally-invasive alternative to open surgery, with reduced recovery times
and potentially improved survival rates." The development of the endovascular
field is chronicled in our video documentary below.
related stories:
"Vascular
Pioneers:
Evolution
of
a
Specialty" -- video
from
Vasculartherapy.Org
June 1, 2006
Dr.
Gabor Rubanyi of Berlex Biosciences Joins Cardium as
Chief Scientific Officer
(source: Cardium Therapeutics, Inc.)
In this company press release, Chairman/CEO Christopher J. Reinhard states, "We
are pleased to welcome Dr. Rubanyi to Cardium Therapeutics. Gabor and I worked
together over the past decade as Berlex and Collateral Therapeutics collaborated
on the discovery, pre-clinical research and clinical development of our lead
product, Generx, for the potential treatment of coronary heart disease. His
extensive experience in angiogenic therapy with Schering/Berlex has been instrumental
in our growth and evolution and he will continue to play a key role as we prepare
to advance Generx into late-stage clinical studies."
May 31, 2006
Cordis
Announces Nationwide Introduction of Breakthrough Devices
to Treat Artery Blockages in Leg
(source: Cordis Corporation)
Cordis Endovascular, division of Cordis Corporation, today announced the nationwide
introduction of two breakthrough devices, FRONTRUNNER® XP CTO and OUTBACK® LTD™ Re-Entry
Catheters to treat artery blockages in the lower leg, a common finding in patients
with diabetes and peripheral vascular disease. Both devices facilitate angioplasty
and stenting in chronic total occlusions (CTO) which are complete or nearly
complete blockages that can lead to surgery or lower leg amputation. Previously,
many patients with CTOs did not have access to these less-invasive procedures
because to treat CTOs with less-invasive methods, a doctor must first cross
through the blockage. The FRONTRUNNER® XP CTO and OUTBACK® LTD™ Re-Entry Catheters
allow physicians to break through complete blockages allowing treatment with
stents or balloons. By using these catheters, patients may avoid having to
undergo difficult surgeries or even amputations. The devices were developed
by Lumend, Inc. of Redwood City, California -- Cordis acquired Lumend last
September.
May 31, 2006
FDA
Approves Xpert™ Study -- First U.S. Study of Stent
Placement to Treat Arterial Blockages Below the Knee
(source: VIVA Physicians, Inc.)
The XCELL Trial expects to enroll 140 patients in 10 centers to study the efficacy
of Abbott Vascular's Xpert self-expanding nitinol stent in treating severe
peripheral arterial disease (PAD) below the knee. The Xpert is currently approved
for use in the U.S. to treat blockages in the biliary ducts that carry digestive
enzymes from the liver. This first-of-kind study in the U.S. will determine
It is estimated that 25 million people suffer from blockages in the arteries
of the lower leg. Principal investigator Dr. James Joye, D.O., of El Camino
Hospital in Mountain View states, "Those with advanced disease may be poor
candidates for surgery and have few treatment options. Many are at high risk
of losing a limb.... This is an important trial to expand patient treatment
options, increase physician awareness and take an important step forward in
preventing amputation."
May 31, 2006
FDA
Clears Boston Scientific System for Removing Clots from Arteries
(source:
Boston
Scientific
Corporation)
The company has received clearance from the U.S. Food and Drug Administration
(FDA) to market its Rio™ Aspiration Catheter. The new device is indicated for
use in the removal of thrombi, or clots, from vessels throughout the body.
Clots can restrict or block blood flow in an artery, creating difficulty or
risk to a patient when undergoing intravascular procedures. The Rio Aspiration
Catheter can be used with other interventional devices, such as those used
to place stents, to open blockages caused by clots in the target area within
a vessel.
May
30, 2006
St.
Francis and Temple University Hospitals Expand Vascular
Imaging Capabilities with New Toshiba Infinix DP-i/FD2
(source:
Toshiba
America
Medical
Systems)
Toshiba's new system, introduced in March, is the only single lab that meets
American College of Cardiology guidelines for both cardiac and peripheral work.
The availability of this system and its adoption reflects the growing trend
in medicine toward treating vascular disease as an entity, whether heart-related
(coronary artery disease) or in the peripheral circulation (legs, kidney, etc.)
-- many patients who have vascular disease in one area often have it elsewhere.
According to Dr. James Burke, director, cardiac catheterization laboratories
and interventional cardiology at Temple University Hospital in Philadelphia, "With
our patients as our top priority, we chose Toshiba's Infinix DP-i/FD2 because
it was clear that the technology would enable us to improve our diagnosis and
treatment capabilities.... We can diagnose and treat all of the cardiac and
vascular problems in our patients without compromising either area. This is
the future of comprehensive cardiovascular care."
May
25, 2006
Making
Patient Safety the Centerpiece of Medical Liability Reform
(source:
Hillary
Rodham
Clinton,
and
Barack
Obama,
New
England
Journal
of
Medicine)
Important enough for the New England Journal of Medicine to make the full text
version available for free, this article discusses the area of liability and
malpractice lawsuits in the context of patient safety. Here is an important
quote:
"Studies show that the most important
factor in people's decisions to file lawsuits is not negligence,
but ineffective communication between patients and providers.
Malpractice suits often result when an unexpected adverse
outcome is met with a lack of empathy from physicians and
a perceived or actual withholding of essential information."
We could not agree more with Senators Clinton
and Obama. All you need to do is read the various postings
on our Patient
Forum about adverse events to see how some physicians' "lack
of empathy" or the "witholding of essential information" upsets
patients and their families. All medical procedures have risks
-- anyone who watches a "doctor show" on TV understands
this. What hospitals and physicians need to do is clearly explain
these risks to the patient. If patients fully and honestly
understand the potential complications of any medical intervention,
and are able to weigh those against the positive outcomes that
the procedure may afford, then both patient and physician are
on the same "side" and everyone is pulling in the
same direction for the desired result.
May 23, 2006
Toshiba
Installs 300th Vantage 1.5T MRI System
(source: Toshiba America Medical Systems)
Toshiba's advantage in this field is its patented Pianissimo™ technology, which
reduces by almost 90% the acoustic noise, identified as the most significant
cause of patient anxiety. Reduction of the noise often eliminates the need
for anti-anxiety medication or sedation during routine scans. In addition,
Toshiba’s SPEEDER parallel imaging technology allows clinicians to perform
exams in a single breath hold, reducing motion artifacts and increasing image
quality. In this company press release radiologist John Mathis from the Center
for Advanced Imaging in Roanoke, Virgina, where the 300th Vantage was installed,
states, “We typically conduct about 4,500 MRI scans a year, and anxiety caused
by system noise is a significant issue for many of our patients. In fact, we
receive countless referrals because we offer the latest in MRI technology with
improved patient comfort.”
related stories:
Magnetic
Resonance
Imaging
and
Angiography
(MRI
/
MRA) -- Angioplasty.Org
May 23, 2006
Campbell
Rogers, M.D., International Expert in Interventional
Cardiology and Vascular Conditions, Named Chief Technology
Officer of Cordis Corporation
(source: Cordis Corporation)
Boston-based Brigham & Women's Hospital's cardiology department is now
batting three-for-three. Last fall Dr. Richard Kuntz left to become a Senior
VP at Medtronic. Then, earlier this month, Dr. Donald Baim announced he
was leaving to become Chief Medical and Scientific Officer for Boston Scientific.
Now Dr. Campbell Rogers is poised to become Chief Technology Officer for Cordis,
Boston Scientific's arch-rival in the drug-eluting stent world.
May 19, 2006
'Guidant'
fading away
(source: Jim McCartney, St. Paul Pioneer Press)
Attendees at this year's Heart Rhythm Society conference may see some Guidant
logos around, but not for long, as Boston Scientific re-brands the company
it acquired earlier this year. Boston Scientific's current tagline: "We
Can and We Will."
May 16, 2006
Recent
Clinical Trials Funded by For-Profit Organizations More Likely
to Report Positive Findings Than Trials Funded by Not-For-Profits
(source:
Journal
of
the
American
Medical
Association
--
JAMA)
A study published in this week's JAMA by Boston researchers from Brigham and
Women's Hospital and Harvard looked at 324 recent cardiovascular clinical trials,
both drugs and devices. They found, not to anyone's great surprise, that in
those clinical trials where there was a financial interest in a positive finding,
the results tended to be positive two-thirds of the time. In those trials sponsored
by a not-for-profit entity (such as a hospital center) the results tended to
be pretty much evenly split.
May 12, 2006
Toshiba's
Technology Contributions Featured on Top Morning Talk
Show "Live With Regis and Kelly"
(source: Toshiba
America
Medical
Systems)
Toshiba Medical and other Toshiba divisions have donated over $80,000 worth
of ultrasound and other equipment to Partners for Healing clinic (PFH) which
serves an uninsured community in Tullahoma, Tennessee, as part of “Live with
Regis and Kelly’s” 16th annual “Mom’s Dream Come True” Mother's Day special.
May 11, 2006
Desensitization
Protocol Overcomes Allergy to Clopidogrel
(source:
Society
for
Cardiovascular
Angiography
and
Interventions
--
SCAI)
A careful desensitization protocol can help patients overcome allergic reactions
to anti-clotting medication critical to preventing new blockages inside coronary
stents, according to a new study being presented at SCAI's annual meeting.
Nicholas E. Walker, MD, a cardiology fellow at the University of Iowa, Iowa
City, states,“We showed we could successfully and safely desensitize patients
who had just recently had a drug-eluting stent placed. That’s a critical population
to manage.” (Read
more...)
May 11, 2006
Federal
Appeals Court Rules in Cardium's Favor Over Boston Scientific
and Arch Development on Patents for the Treatment of
Heart Disease
(source:
Cardium
Therapeutics,
Inc.)
An court ruling back in March prompted
Boston Scientific and Arch Development to an appeal. That appeal has been rejected,
reports Cardium Therapeutics, which means that this specific technology for
delivering genetic material via catheter to the heart to promote angiogenesis
cannot be patented by Boston Scientific and is the exclusive property of Cardium.
May 11, 2006
Boston
Scientific Announces Appointment of Donald Baim, M.D.
as Chief Medical and Scientific Officer
(source: Boston Scientific Corporation)
Dr. Baim, currently Senior Physician at the Brigham and Women's Hospital in
Boston, will be replacing Dr. Mary E. Russell as of July.
May 9, 2006
Toshiba
Achieves Educational Milestone for Unique Physican-to-Physician
Speakers Bureau Program
(source: Toshiba America Medical Systems)
The company has now enrolled more than 2,500 cardiologists and radiologists
in its education and training programs to help expand the knowledge base among
medical professionals regarding the latest diagnostic imaging modalities, and
64-slice CT technology in particular.
related stories:
MultiSlice
Computed
Tomography
(MSCT) -- Angioplasty.Org
May 9, 2006
Safety
Risk of Multislice CT Angiogram Compared to Cardiac Catheterization
Two articles in the recent Journal of the American College of Cardiology, published
online on April 17, address the debate of how non-invasive MultiSlice Computed
Tomography (MSCT) imaging compares to standard angiography (cardiac catheterization)
on the issue of safety. Whether or not the radiation dosage of one procedure
is higher than another is only one part of the safety equation. (Read
more...)
May 8, 2006
Toshiba
Ships 600th Aquilion 64-Row Detector System Worldwide
(source: Toshiba America Medical Systems)
In their press release, Toshiba states that this is "a significant milestone
for the company’s CT business, which has installed more than 17,000 CT systems
in hospitals, imaging centers and medical group practices worldwide, including
the Johns Hopkins University School of Medicine, Beth Israel Deaconess Medical
Center, Shands at the University of Florida and Cardiovascular Institute of
the South (CIS). According to Peter S. Fail, M.D., cardiologist, CIS, growing
patient awareness about advances in diagnostic imaging and physician demand
for cutting edge imaging technologies has encouraged many healthcare facilities
to acquire 64-slice CT systems to remain competitive and better serve patients."
May 8, 2006
Dr.
Julio Palmaz, Inventor of Stent Widely Used in Coronary
and Peripheral Disease, Inducted Into National Inventors
Hall of Fame
(source:
Cordis
Corporation)
Dr. Julio Palmaz of the University of Texas Health Science Center at San Antonio
was inducted into the National Inventor's Hall of Fame on Saturday. Dr. Palmaz
was recognized for the invention of the balloon-expandable stent, a revolutionary
medical device that he worked on in his garage with materials from Radio Shack
et al, inspired by Gruentzig's invention of the angioplasty balloon. Palmaz's
stent concept was not confined only to the coronary arteries, but was the basis
for the first AAA endograft, among many other devices (Palmaz, worked with
and joined fellow Argentinian Juan Parodi, scrubbing in and deploying the stent
which he co-invented in the first AAA case done in Buenos Aires -- for more,
see our documentary Vascular
Pioneers: Evolution of a Specialty). Dr. Palmaz is the holder of
numerous important patents in the medical device field.
related stories:
"Origins
of
the
Palmaz-Schatz
Stent"
Dr. Richard Schatz, co-inventor of the Palmaz-Schatz stent that revolutionized
the treatment of coronary artery disease, discusses Gruentzig's influence on
Palmaz and himself -- Angioplasty.Org (RealVideo clip -- RealPlayer required)
Dr.
Julio Palmaz, 2006 Inductee profile -- National
Inventors Hall of Fame
May 3, 2006
Vasomedical
Sells Its 1,000th EECP Therapy System
(source:
Vasomedical,
Inc.)
The company has announced the sale of its 1,000th EECP® therapy system to St.
Francis Hospital, The Heart Center® in Roslyn, New York. "St. Francis Hospital,
The Heart Center continues to demonstrate its commitment to providing high-quality
services to patients with cardiovascular disease by offering EECP therapy with
Vasomedical's state-of-the-art Lumenair™ system," stated Dr. Justine Lachmann,
director of the Congestive Heart Failure Program at St. Francis Hospital. This
milestone comes at a difficult time for the company. Medicare recently declined
to expand the reimbursement indications for EECP therapy, although Vasomedical
is hoping to appeal that once their PEECH study is published. As a result the
company's stock price has fallen and it has received a delisting notice from
NASDAQ.
related stories:
Vasomedical
Receives
Nasdaq
Delisting
Notice -- Vasomedical,
Inc.
Enhanced
External
Counterpulsation
(EECP)
--
Discussion
Forum -- Angioplasty.Org
May 2, 2006
Americans
Less Healthy than English
(source:
Journal
of
the
American
Medical
Association)
How can this be? The United States spends almost 2 1/2 times more per person
on healthcare than the United Kingdom. This study of individuals in late middle
age (55-64 years old) was done by London researchers and appears in the May
3 issue of JAMA -- it is sure to raise controversy. The study compared data
from both countries and found some interesting facts. 12.5% of Americans suffer
from diabetes, double that of the U.K., and high blood pressure is 10% higher
in the U.S. Across the board, the British suffer less from diabetes, hypertension,
heart disease, heart attack, stroke, lung disease and cancer. As for "vices",
smoking was similar is both countries (about 20% in this age group), heavy
alcohol consumption was higher in the U.K., obesity much higher in the U.S.
As one might expect, lower income groups in both countries were less healthy
than those in higher income brackets. But a very interesting statistic -- the
differences in health were so great between the two countries that the high
income group in the United States had the same health profile as the low income
group in Britain. So access to healthcare does not seem to be the cause of
this difference, because the wealthiest Americans certainly have access to
healthcare. Note also that the study was limited to non-Hispanic whites in
both countries to eliminate a bias to health-related issues in the black or
Latino populations in both countries.
May 1, 2006
Medtronic
Receives FDA Approval for Micro-Driver Stent
(source: Medtronic, Inc.)
The Micro-Driver is a bare metal cobalt alloy system designed specifically
to perform in small vessels and tortuous anatomies, and the first bare metal
stent for small vessels with an indication for new or untreated vessels (a
de novo indication). According to Dr. Michael Ball of St. Vincent Hospital
in Indianapolis, "This stent fulfills an essential need that we have in cardiology....
When you do small vessel stenting, you want the stent to be small and you want
it to be flexible. That's the whole idea behind Micro-Driver. It conforms well
to the anatomy and allows you to take care of more lesions. I think more patients
should be able to benefit from this technology." Medtronic states in its press
release that even in the drug-eluting stent era, many physicians choose bare
metal stents for patient care in small-vessel cases, and the Micro-Driver stent
offers them an important treatment option. "
April 27,
2006
Medtronic
Receives CE Mark For New Carotid Stent And Filter
(source: Medtronic, Inc.)
The new Medtronic Exponent® RX Self-Expanding Carotid Stent is a tiny metal
scaffold that is used to prop open a blocked artery and restore adequate blood
flow. While not yet approved for use in the United States, it is part of the
Medtronic Carotid Solution, which also includes the Interceptor™ PLUS Carotid
Filter System. “Carotid artery disease and stroke affect hundreds of thousands
of people each year, and carotid stenting offers patients a less-invasive treatment
option when compared to open surgery,” said Sean Salmon, vice president and
general manager of Medtronic Vascular’s Coronary and Peripheral businesses. “Products
like the Exponent RX carotid stent and the Interceptor PLUS filter are innovatively
engineered to provide effective therapy even in challenging and complex anatomies
without compromising safety.”
April 24,
2006
Dr.
Tom Ferguson, Who Urged Self-Education, Dies at 62
(source:
Nadine
Brozan,
New
York
Times)
"Tom Ferguson, who spent his life as a physician persuading people to take
the reins of their own health care and to use the Internet as a powerful tool
in that quest, died on April 14 in Little Rock, Ark.". Dr. Ferguson was
a pioneer and foresaw the wave of patient interest in the internet. Besides being
the health and medical editor of the famed 60's and 70's Whole Earth Catalog,
he also served as senior research fellow at the Pew Internet and American Life
Project whose reports we often quote on Angioplasty.Org. regarding patient use
of the Internet for health information. The article sums up his philosophy (which
we share): "He urged patients to educate themselves and share knowledge
with one another, and urged doctors to collaborate with patients rather than
command them."
April 24, 2006
Aspirin
Dispute Is Fueled by Funds Of Industry Rivals
(source:
David
Armstrong,
Wall
Street
Journal
$$)
Are some heart patients resistant to aspirin? This story (WSJ subscribers only
-- sorry) explores the various forces in the pharmaceutical industry that have
generated studies and articles both for and against this idea, with one side
claiming that the benefits of an inexpensive medication like aspirin are being
questioned by doctors who have financial relationships with companies that
make the tests for "aspirin resistance" or with pharmaceutical firms
that make more expensive antiplatelet medications. Highly-regarded and often
controversial Dr. Eric Topol, formerly of the Cleveland Clinic, weighs into
the mix as well.
April 20, 2006
Boston
Scientific Receives FTC Antitrust Approval for its Combination
with Guidant
(source:
Boston
Scientific
Corporation)
The long-awaited and much-anticipated combination of Boston Scientific and
Guidant has now cleared its last regulatory hurdle and the deal is set to close
tomorrow, Friday, April 21, 2006.
April 11, 2006
Toshiba
Names General Manager
(source: Toshiba America Medical Systems, Inc.)
The company has named diagnostic imaging industry veteran, Lawrence Dentice,
general manager, senior vice president and an officer of the company. Dentice
has been Toshiba’s vice president, sales, since he joined the company in 2002.
Former general manager, Edwin Lodgek, has left the company.
April 11, 2006
Boston
Scientific Receives European Commission's Antitrust Clearance
for Guidant Transaction
(source:
Boston
Scientific
Corporation)
The European Union Commission has given its approval to the combining of Boston
Scientific and Guidant, a week earlier than the deadline. This leaves the FTC
to approve the deal.
April 10,
2006
Corautus
Announces Termination of Patient Enrollment in GENASIS
Severe Angina Clinical Trial
(source: Corautus Genetics Inc.)
Based on the recommendation of its independent Data Monitoring Committee (DMC),
the company has announced termination of patient enrollment in its GENASIS
Phase IIb clinical trial. The trial was suspended
on March 14 due to several adverse events which the company stated did
not seem to be related to the biologic VEGF-2. The delivery system for the
genetic material is manufactured by Boston Scientific with whom Corautus established
a strategic
alliance and financial relationship in 2003. Given those events, and review
of the current data, the DMC saw very little chance for significant efficacy
as to the primary endpoint relative to the safety risk signal it saw. Corautus
will continue to collect data on patients enrolled to date for six months,
at which time the data will be analyzed to determine efficacy. Safety data
will be montiored through one year. The company's stock plunged over 67% after
the news. What is not clear is the cause of the adverse events; this information
will be very important for the entire field of genetic research into treatment
of coronary artery disease.
April 10,
2006
Toshiba
America Medical Systems Becomes American College of Cardiology
Foundation "Imaging Champion"
(source: Toshiba America Medical Systems, Inc.)
Toshiba has announced major support of a series of educational programs presented
over the next year by the American College of Cardiology Foundation that will
bring together clinical experts in ultrasound, nuclear cardiology, angiography,
computed tomography and magnetic resonance imaging to present the current state-of-the-art
in imaging and update medical professionals in the field. Of note is the inclusion
of Cardiac CT for the first time in several of the programs, indicating the
growing interest in this imaging modality.
April 5, 2006
Boston
Scientific Signs Agreement Resolving Outstanding Antitrust
Issues with FTC Staff
(source:
Boston
Scientific
Corporation)
In this press release the company states that it has entered into an agreement
with the staff of the Federal Trade Commission. The agreement contains consent
orders and resolves all outstanding antitrust issues with the FTC staff, although
the commissioners still need to approve the agreement. Furthermore, the company
states that "on or about April 7, it will voluntarily withdraw and re-file
its notification under the Hart-Scott-Rodino Antitrust Improvements Act of
1976...to ensure that the FTC Commissioners have adequate time to review the
executed consent decree." The H-S-R Act sets a 30-day waiting period after
filing. However, Boston Scientific states that they expect to close the transaction
by mid-April, about 10 days from today. Currently, Boston Scientific is paying
a "late fee" to Guidant shareholders for every day past April 1 that
the deal is not closed -- estimated at $4.5 million/day. The stock closed at
21.69 today, down 7% since Friday and below the "collar" inside of
which Guidant shareholders get additional shares of Boston stock.
April 5, 2006
Cardium
Provides Updated Summary of Generx Clinical Program for
Potential Treatment of Coronary Heart Disease
(source: Cardium Therapeutics, Inc.)
The company provides in this press release an updated summary of its clinical
development of Generx, a product candidate for the potential treatment of patients
with myocardial ischemia and recurrent angina associated with coronary heart
disease.
April 4, 2006
Cordis
Corporation To Develop Global Cardiac And Vascular Institute
(source:
Cordis Corporation)
Cordis Corporation announced today plans to develop a world-class global cardiac
and vascular institute that will provide physicians and other health care providers
with educational and clinical resources designed to help advance the understanding
and treatment of a broad range of cardiac and vascular conditions, including
coronary artery disease and stroke. The institute will also enhance knowledge
around the management of congestive heart failure, adult cell therapy and advanced
cardiac imaging, mapping and ablation.
March 31, 2006
Boston
Scientific and Guidant Shareholders Each Approve Combination
(source: Boston Scientific Corporation / Guidant
Corporation)
Shareholders of the two companies approved the combination today. Once the
European Union and Federal Trade Commissions approve the deal, the two companies
will become one.
March 30,
2006
Parma
Community Hospital Enhances Patient Care, Optimizes Workflow
and Expands Clinical Capabilities with Toshiba's New
Infinix-i Series
(source: Toshiba America Medical Systems, Inc.)
The company announces installation of its latest flat panel detector vascular
and cardiac imaging system in Parma (Ohio) Community Hospital. A company spokesperson
says, "The Infinix CC-i FPD is uniquely designed to meet the select needs
of clinicians conducting complex cardiac imaging procedures. In addition to
the system’s unparalleled image quality and lower dose, it improves diagnosis
and treatment while maintaining patient safety."
March 29,
2006
Cardium
Announces Patent Wins Over Boston Scientific and Arch
Development Corporation
(source:
Cardium
Therapeutics,
Inc.)
Cardium Therapeutics of San Diego has announced patent decisions which protect
and distinguish their method of delivering gene therapy to the heart in order
to promote angiogenesis. The company states that the therapy is meant to treat
the very small blood vessels in the coronary circulation, vessels too small
to balloon or stent open, one reason that patients may continue experiencing
angina even after their main coronary arteries are opened up either mechanically
or surgically.
March 28,
2006
Borgess
Medical Center Enhances Cardiac Capabilities for Advanced
Patient Care
(source: Toshiba America Medical Systems, Inc.)
In this press release, the company announces installation of five new catheterization
labs in this Kalamazoo Michigan hospital, in addition to the existing Aquilion
CT scanner. "According to Tim Fischell, M.D., director of cardiac research
at the Borgess Heart Center for Excellence, incorporating diagnostic imaging
into the center’s practice has dramatically improved the level of cardiac care
they are able to deliver to patients. “CVD continues to be a major problem
in our communities, and advances in diagnostic imaging have had a significant
impact in diagnosing disease of the heart at its earliest stages and preventing
death.” Dr. Fischell is the co-inventor of the BX Velocity stent, which forms
the foundation of Cordis' Cypher drug-eluting stent.
March 27, 2006
Medtronic
Receives FDA Approval for New AneuRx® AAAdvantage™ Abdominal
Aortic Aneurysm Stent Graft System
(source:
Medtronic
Inc.)
Medtronic has achieved approval for its next-generation, improved AAA stent
graft for repairing abdominal aortic aneurysms. Approximately 1.2 million people
in the United States have an AAA condition. Only about 15 percent are ever
diagnosed and less than half of those are ever treated. The danger of an AAA
is that the bulging of the arterial wall in the aorta, the largest artery in
the body, will lead to rupture, which is fatal in most cases. The standard
surgical treatment for repairing this kind of aneurysm is being replaced in
certain patients by an endovascular approach where, much like in coronary angioplasty,
a catheter with a special stent is inserted through the femoral artery in the
groin, and threaded up into the aorta, where a balloon expands the stent graft
and is then withdrawn. The first endovascular AAA repair done in the U.S. was
in the early 90's (the story of that procedure, invented by Dr. Juan Parodi
of Argentina, can be seen in Chapter
Four of our video "Vascular Pioneers" -- RealPlayer required,
or buy the DVD).
and done in the U.S. by Dr. Frank Veith and Dr. Michael Marin along with Parodi
at Montefiore Hospital in New York).
March 27,
2006
European
Union Delays Decision On Boston Scientific Buy Of Guidant
(source:
Dow
Jones
Newswires)
The EU had originally said it would make its decision by March 31 on whether
or not to approve Boston Scientific's purchase of Guidant. The company stated
in a press release late last week that the EU decision might be delayed due
to certain confidential commitments made to the Commission to resolve anti-trust
issues, but that the decision would not be delayed past April 13 and might
be made prior to then. However, the EU Commission today stated that that the
new deadline is April 18 and, according to Reuters, has said "the investigation
was no longer following its simplified procedure." Last week the Wall
Street Journal reported that the five-day delay was due to the long Easter
weekend holiday as it is celebrated in Europe. Part of the offer to Guidant
shareholders was that Boston Scientific would pay an additional fee/share for
every day past March 31. This comes to about $4.5 million/day.
related stories:
EU
sets
new
deadline
for
Boston/Guidant
probe -- Reuters
European
Holiday
May
Cause
Small
Boston
Sci/Guidant
Delay -- Wall
Street
Journal
($$)
Boston
Scientific
Makes
Antitrust
Commitments
To
European
Commission -- Boston
Scientific
Corp.
(Mar
23)
March 20, 2006
Institutional
Shareholder Services Recommends That Shareholders of Boston
Scientific and Guidant Approve Combination
(source: Boston Scientific Corporation)
Shareholders of Guidant and Boston Scientific are scheduled to vote on Friday,
March 31 whether to approve the purchase of Guidant by Boston. In this press
release, Boston Scientific states that ISS, a "leading U.S. independent
proxy advisory firm" has recommended that shareholder vote yes.
March 16, 2006
American
Heart Association Statement: Patient Guidance Based on
Results of the CHARISMA Trial
(source:
American
Heart
Association)
Dangers
of
Stopping
Clopidogrel
(Plavix®)
for
Patients
with
Stents
and
Certain
Other
Conditions
(source:
American
College
of
Cardiology)
In a signficant display of patient e-Powerment, the reaction among patients
to the very misleading news coverage of the CHARISMA trial presented at the
ACC last Sunday was so explosive that both the AHA and ACC have now seen fit
to issue a guidance to patients, saying precisely what we at Angioplasty.Org
warned about three days earlier:
Stent Patients! Don't Stop Taking Your Plavix and Aspirin without consulting
with your cardiologist! As we predicted in our editorial
blog entry, cardiologists have been fielding questions all week about whether
or not patients should be stopping their Plavix and aspirin regimen. As reflected
in the AHA & ACC guidances (actually the ACC calls it a "Public Health
Alert") and the AP article below, doctors are saying "don't stop"!
related stories:
Doctors
Urge
Not
to
Stop
Taking
Plavix -- Associated
Press
Plavix
and
Aspirin:
Don't
Stop
Taking
Your
Meds -- "Voice
in
The
Ear":
The
Stent
Blog
March 15, 2006
FDA
Clears Innovative Cardiac Navigation Technology
and
NOGA® Cardiac
Navigation System Helps Researchers Deliver Stem Cells to the Heart
(source: Cordis Corporation)
A novel navigation system for 3-D imaging inside the coronary anatomy was approved
today by the FDA, and also figures into an experimental stem cell research
project to help patients with weak heart who are not candidates for surgery
or stenting.
 March
14, 2006 March
14, 2006
256
Multislice CT Angiography One Beat Whole Heart Scan:
First Clinical Data at ACC
(source:
American
College
of
Cardiology)
Developed in Japan by Toshiba Medical, this experimental CT multislice scanner
was able to image the entire heart in a single heartbeat in 1.5 seconds.
March
14, 2006
Corautus
Genetics Inc. Announces Developments in Clinical Trial
and Public Offering
(source:
Corautus
Genetics
Inc.)
...and the developments they announced aren't positive ones. Corautus has been
conducting the GENASIS clinical trial of its VEGF-2 biologic, in conjunction
with Boston Scientific and using Boston's Stiletto™ catheter -- the idea
is to deliver cells to the heart to improve the circulation through VEGF (vascular
endothelial growth factor) as a treatment for severe angina. Three patients
recently reported serious adverse events of pericardial
effusion which, they stress, does not seem related to the biologic.
Boston Scientific requested that Corautus voluntarily suspend the trial while
a short review into what the problem might be takes place. Additionally, in
light of this development, the company and its underwriters agreed to cancel
its upcoming public offering of stock.
related stories:
Corautus
halts
chest
pain
trial,
stock
falls -- Reuters
Corautus
Halts
Chest
Pain
Drug
Trial -- Associated
Press
Angiogenesis -- Angioplasty.Org
 March
13-14, 2006 March
13-14, 2006
Intensive
Statin Therapy May Partially Reverse Plaque Build-up
in Arteries
and
Many High
Risk Patients Do Not Receive Cholesterol-Lowering Therapies
(source: American College of Cardiology)
The two press releases above from the American College of Cardiology meeting
in Atlanta tell a story. The first discusses the ASTEROID trial, presented
by Dr. Steve Nissen of the Cleveland Clinic (also President-Elect of the ACC).
In this study, his team looked at a group of 500+ patients. Nissen used intravascular
ultrasound (IVUS), an imaging technology that actually views the coronary
artery from the inside. IVUS allows the most precise measurement of the diameter
of the artery, including the thickness of layers of plaque. The patients were
studied at the beginning at ASTEROID, then received 40 mg daily of the statin
drug rosuvastatin (Crestor®, manufactured by AstraZeneca). 24 months later,
the patients were restudied. Their LDL (so-called bad cholesterol) had gone
down 53% and their HDL (good) rose almost 15%. But news was generated by the
fact that upon IVUS measurement, not only had the progression of coronary artery
disease been slowed down -- it was actually reversed. The plaque was reduced
between 7-9%. Dr. Nissen has stated that this shows very low LDL levels can
reduce plaque -- whether this can actually reduce heart attacks or mortality
will need to be determined by a further study. But ASTEROID certainly represents
a significant piece of data in the fight against heart disease.
The second press article's headline speaks
for itself -- especially ironic in light of the ASTEROID study.
Although, like all drugs, statins have some side-effects, it
has been known for some time that statins are of benefit to
patients at high risk for heart disease. The ASTEROID results,
that statins can reverse plaque build-up, are just the most
dramatic and recent example of these benefits. So the study
results of 142,000 patients who were classified "at risk" is
even more troubling: the majority of these patients were not
prescribed statin therapy during the first year after diagnosis.
States Richard H. Chapman, Ph.D., of ValueMedics Research and
lead author of the study, "Statins are the standard of care
for this group of patients, and they should be prescribed along
with antihypertensive therapy. Failing to do so may restrict
patients’ access to potentially life-saving treatment." The
conclusion is unfortunately also not new. A year ago, Angioplasty.Org
reported on a study
out of Stanford by Dr. Jun Ma which came to the
same conclusion.
A Google News search found 63 articles about
the ASTEROID study, but only one reprinted press release about
the second study. Angioplasty.Org will soon be posting an exclusive
interview with Dr. Ma of Stanford. Register
for our newsletter to be informed when it is online.
 March
13, 2006 March
13, 2006
Stent
Patients -- Don't Stop Taking Your Meds: Media Reports
About CHARISMA Confuse Patients:
(source: "Voice
in
the
Ear" Stent
Blog)
Many headlines and news reports are warning that taking Plavix and aspirin
together could be a dangerous combination. These results apply to patients
who have no symptoms of arterial disease, but is a very misleading warning
for angioplasty/stent patients. Read why in our Editor's
Blog.
related stories:
Results
of
CHARISMA
Study
--
Press
Release -- Bristol-Myers
Squibb;
sanofi-aventis
Plavix
and
Aspirin
Dosage
After
Stent -- Angioplasty.Org
Forum
CHARISMA:
No
benefit
of
long-term
clopidogrel
in
stable
disease -- theheart.org
(subscription
required)
 March
12, 2006 March
12, 2006
Angiomax® (bivalirudin)
Monotherapy Superior to Heparins Plus GP IIb/IIIa Combination
for Net Clinical Outcome in ACUITY Trial
(source:
The
Medicines
Company)
One of the problems cardiologists have in treating patients who are on their
way to the cath lab for either a diagnostic angiogram, or possible an angioplasty/stent
placement, is the use of anti-thrombin drugs, blood thinners, etc. It is necessary
to administer these medications to ensure a safe interventional procedure --
one where blood clots don't form. But these drugs also tend to cause bleeding,
both internally and also at the femoral access point (hematomas, etc.). The
ACUITY study, led by Dr. Gregg Stone, has found that a different drug, one
that is less likely to cause bleeding, actually performs better than the currently
used protocol of Heparin combined with GP IIb/IIIa inhibitors. The drug is
Angiomax, or bivalirudin, and it was tested by itself (montherapy) and in combination
with the Heparin and GP IIb/IIIa inhibitors. Monotherapy showed superior results,
while Angiomax combined with Heparin and GP IIb/IIIA inhibitors was about the
same as the Heparin and GP IIb/IIIA therapy alone. Dr. Stone states, "Previous
ACS trials have added drug on top of drug to achieve better efficacy, sacrificing
safety along the way. The ACUITY trial showed that Angiomax alone is as effective
as more complicated dual drug regimens, and results in significantly less bleeding,
which means improved outcomes for patients."
related stories:
Angiomax
better
than
other
blood
thinners-study -- Deena
Beasley,
Reuters
Medicines
Shares
Up
on
Angiomax
Study -- Associated
Press
March 10, 2006
Initial
Results of PROGRESS–AMS Study Confirm Safety and Feasibility
of Absorbable Metal Stent Technology in Human Coronary Vessels
(source:
Biotronik
AG)
Biotronik announces the results of a safety and feasibility trial for its absorbable
stent. No cardiac deaths, myocardial infarctions, or stent thromboses were
observed. And while the restenosis rate that is not yet "in line with
clinical standards", information was gained to fine-tune the degradation
process so future trials will hopefully show good restenosis results. Most
importantly, the stent was shown to have completely disappeared (using IVUS
imaging). Angioplasty pioneer Professor Raimund Erbel, who is the principal
investigator in this trial, stated, “The AMS is not just another stent. It
represents a revolutionary leap forward in vascular intervention treatment.
The AMS provides the necessary short term scaffolding needed to support the
vessel during the healing process and is then absorbed, avoiding the long-term
mechanical stress of current permanent stent technologies.”
March 7, 2006
KLAS
Survey Ranks Toshiba's Aquilion 64-Slice CT System Top
Among Leading Medical Imaging Technology Vendors
(source:
Toshiba
America
Medical
Systems,
Inc.)
The survey included more than 300 healthcare professionals, including imaging
directors and managers, C-level executives and physicians on survey categories,
including service; ability to deliver product; upgrade path; scanner reliability;
end- user presentation; cost and value; technology and architecture; post-processing
capabilities; and PACS (picture archiving and communication systems) integration.
In addition to Toshiba’s top distinction in overall vendor ratings, the survey
revealed that Toshiba scored highest in four categories, including cost and
value, technology and architecture, post processing capabilities, and integration
into existing PACS solutions.
March 7, 2006
Baxter
Commences First-Of-Its-Kind, Phase II Adult Stem Cell
Trial in U.S. In Patients With Severe Coronary Artery
Disease
(source:
Baxter
Healthcare
Corporation)
This second phase follows a smaller Phase I in which safety of the treatment
was demonstrated. In this treatment, patients' own stem cells are reinjected
into areas of their heart with critically poor circulation. Only 24 patients
were treated, but 16 reported feeling better and having increased exercise
capability. Douglas Losordo, M.D., chief of cardiovascular research at Caritas
St. Elizabeth's Medical Center in Boston, will be the lead investigator of
this second phase. He states, "This trial is the culmination of nine years
of research suggesting the potency of CD34+ cells for treating heart disease....
We are delighted that we are on the threshold of the next important phase of
testing for this unique autologous stem cell therapy.”
March
5, 2006
A
64-slice heart X-ray, sans catheter, is catching on
quickly at Sequoia Hospital
(source:
Sam
Whiting,
San
Francisco
Chronicle)
This feature, about a community hospital in Redwood City, discusses the benefits
of the new 64-slice CT scan technology.
March 4,
2006
Shop
and compare hospital care
(source: Bob Moos, Dallas Morning News)
This report from the Dallas Morning News shows how healthcare consumers on
the internet are using websites to give hospitals a checkup and make decisions
on where to go. From the article: " One of the most popular Web sites
is Medicare's, which shows how well 4,200 hospitals follow generally accepted
guidelines for treating heart attacks, heart failure and pneumonia and preventing
surgical infections."
related site:
Hospital
Compare -- Centers
for
Medicare
and
Medicaid
Services
(U.S.
Dept.
of
Health & Human
Services)
March 2, 2006
Boston
Scientific and Guidant Proposed Merger Registration Statement
Declared Effective
(source: Boston Scientific Corporation)
In this company press release, Boston Scientific states that the merger agreement
has been accepted as "effective" by the U.S. Securities and Exchange
Commission (SEC) and that they expect to close the week of April 3, pending
review and approval from the FTC and the European Union
February 14,
2006
WomenHeart
and the Society for Women’s Health Research Unveil
The 10 Q Report
(source: Society for Women's Health Research)
WomenHeart and SWHR surveyed experts in the cardiovascular field and asked
them to identify the top ten unanswered research questions. They cover effectiveness
of risk assessment and diagnostic tools, the differences in risk and in effectiveness
of therapies for men and women, and the need for improved understanding of
cardiovascular disease in women. Two of the questions noted are: (1) Why do
women receive significantly fewer referrals for advanced diagnostic testing
and treatments for heart disease than men, and how can the referral rate for
women be increased? and, (2) Why are women age 50 and younger more likely to
die following a heart attack than men of the same age?
related stories:
The
Complete 10 Q Report -- Society for Women's Health
Research
February 14, 2006
CT
Scanner reveals hidden threats
(source: San Jose Mercury News)
Story about a 62-year-old California man who volunteered for a test of a new
64-slice CT scanner at Kaiser Permanente. Although he had no history, indications
or symptoms of heart disease, the scan revealed extensive blockage in one of
his coronary arteries. The blockage was addressed later with a stent.
related stories:
Image
Makers
--
The
Promise
of
Cardiac
CT
Angiography -- "Voice
in
The
Ear":
The
Stent
Blog
February 7, 2006
Boston
Scientific Announces Results for Year and Fourth Quarter
Ended December 31, 2005
(source:
Boston
Scientific
Corporation)
The company announced financial results from the last quarter and the full
year. While profits were up, sales of its Taxus stent were down in the last
quarter -- 12% worldwide and 21% in the U.S. The company also stated that it
expected to complete its acquisition of Guidant by the end of March, pending
regulatory approval.
related stories:
Boston
Scientific
Net
Rises
Despite
Drop
in
Stent
Sales -- Wall
Street
Journal
$$
Boston
Scientific
Profit
Up,
Sales
Slide -- Mark
Jewell,
Associated
Press
February 3, 2006
Statement
of Boston Scientific CEO Jim Tobin on FDA Meeting
(source:
Boston
Scientific
Corporation)
Boston Scientific CEO Jim Tobin met with the F.D.A. today in an attempt to
resolve issues surfaced by the very
stern warning letter sent last week which, among other things, froze future
approvals of the company's devices. Shortly before 4:00pm, the company issued
this short statement. The good news for the company is that the FDA is not
going to take further actions and promises to monitor and meet with company
officials in a timely manner. The company stated it is committed to resolving
the issues on an "aggressive timeline", however, what that timeline
might be was not discussed, nor was the lifting of restrictions on new approvals.
These will most likely be hot topics to be discussed in detail during next
Tuesday's earnings conference call.
related stories:
Device
Maker
Moves
to
Appease
the
F.D.A. -- Barnaby
J.
Feder,
New
York
Times
Boston
Scientific's
CEO
meeting
with
FDA
today
about
quality
problems -- Avram
Goldstein,
Bloomberg
Left
to
One's
Own
Devices:
Adverse
Event
Reporting -- "Voice
in
The
Ear":
The
Stent
Blog
Boston
Scientific
holders
await
comment
after
FDA
talk -- Reuters
Tracking
of
medical
devices
taxes
FDA -- Star
Tribune
(Minneapolis)
FDA
Statement
Regarding
Boston
Scientific
Corporation -- U.S.
Food
and
Drug
Administration
February 1,
2006
A
Gender Difference In Heart Disease
(source:
Rob
Stein,
Washington
Post)
A report on the National Institute of Health's WISE (Women's
Ischemia Syndrome Evaluation) study, which found that the disease process of
atherosclerosis may manifest itself differently in some women. The article
states: "Instead of developing obvious blockages in the arteries supplying
blood to the heart, these women accumulate plaque more evenly inside the major
arteries and in smaller blood vessels, the researchers found. In other cases,
their arteries fail to expand properly or go into spasm, often at times of
physical or emotional stress."
related stories:
Gender
Differences
in
the
Management
and
Clinical
Outcome
of
Stable
Angina -- Circulation,
American
Heart
Assoc.
The
American
Heart
Association's
journal
is
making
this
article
in
the
current
issue
available
for
free
--
it
is
a
European
study
showing
a
significant difference in
the
way
women
with
stable
angina, "the
most
prevalent
manifestation
of
coronary
artery
disease".
are
diagnosed
and
treated
when
compared
to
men.
The
study
states, "Women
with
confirmed
coronary
disease
were
less
likely
to
be
revascularized
[opening
up
the
blocked
artery,
ed.]
than
their
male
counterparts
and
were
twice
as likely to
suffer
death
or
nonfatal
myocardial
infarction
during
the
1-year
follow-up
period".
[Note:
the
full
study
is
in
PDF
form;
an
abstract
can
be
found here.)
Women
Are
Said
to
Face
Hidden
Heart
Disease
Risk -- Denise
Grady,
New
York
Times
January 28, 2006
Boston
Scientific Shares Tumble, May Devalue Deal
(source: Wall Street Journal $$)
Echoing what has been wafting over the financial markets all day Friday, the
Wall Street Journal reports on how the negative fallout from the FDA warning
may affect the Boston Scientific - Guidant acquisition, as BSC's stock price
goes lower and lower.
related stories:
Left
to
One's
Own
Devices:
Adverse
Event
Reporting -- "Voice
in
The
Ear":
The
Stent
Blog
Boston
Scientific's
Slippery
Slope -- Amy
Barrett,
BusinessWeek (Feb
1)
January
27, 2006
Corporate
Warning Letter to Boston Scientific
(source:
U.S.
Food & Drug
Administration)
The FDA delivered a strongly-worded warning letter to Boston Scientific about
its inaction in resolving "serious deficiencies" that were found
during previous inspections. In fact, the word "serious" is used
15 times throughout the letter. There are a number of issues addressed, covering
a number of Boston Scientific's facilities and products, including the major
facility in Maple Grove, where the Taxus stent is produced. The thrust of the
letter is that these issues have not been resolved and the FDA is requesting
an immediate meeting with senior management, not factory executives, because
the agency sees the problem as a corporate-wide failure. Several of the problems
have to do with the company failing to file a number of adverse reports to
the MDR database in a timely manner. The warning letter states that the FDA
could take action without further notice, such as seizing product inventory,
obtaining a court injunction against marketing the product, etc. The letter
also states that no product approvals will be made until these deficiencies
are resolved. The letter is strongly worded because, as the FDA indicates,
these issues were surfaced months ago and the matters have not been satisfactorily
resolved. The letter was issued the day that Boston Scientific and Guidant
penned their acquisition agreement, although not made publicly available until
late yesterday, and analysts are mixed over the implications of this news.
Jan Wald, a health care analyst at A.G. Edwards stated to the Pioneer Press, "This
could put a scare into investors, who could force the stock price down — and
that could end up unraveling the (Guidant) deal." According to the Boston Globe,
Paul LaViolette, COO and other executives will be meeting on February 3 to
discuss the warning letter and they believe they will resolve the issues, although
full resolution will depend on changes being made and new inspections, a process
that will take months and could possibly delay approval of Boston Scientific's
much-anticipated 2nd generation drug-eluting stent, the Liberte.
Boston's stock closed down over 6.5% today.
related stories:
Boston
Scientific
Announces
Two
Communications
From
FDA -- Boston
Scientific
Corporation
FDA warns Boston Scientific over broad problems -- Reuters
FDA
Criticizes Boston Scientific Over Quality-Control Deficiencies -- Wall
Street Journal $$
F.D.A.
Warns
Device
Maker
Over
Safety -- New
York
Times
FDA
issues
Boston
Scientific
stern
quality-control
warning -- Boston
Globe
Boston
Scientific
told
to
fix
plant
flaws -- Twin
Cities
Pioneer
Press
January 25, 2006
Boston
Scientific and Guidant Announce Signing of Merger Agreement
Valued at $27 Billion
(source:
Boston
Scientific
Corporation
/
Guidant)
Guidant has accepted Boston Scientific's higher bid, rejected Johnson & Johnson's
year-long pursuit, and entered into an agreement for $27 billion.
January 25, 2006
Deadline
Passes Without J&J Raising Bid
(source:
Associated
Press)
The midnight deadline passed with no word from Johnson & Johnson on a counter-bid
for Guidant, so Guidant's Board must decide by the close of business on Wednesday
whether to accept Boston Scientific's significantly higher bid, which is likely.
Speculation will now be turned towards what company Johnson & Johnson may
look to acquire. Of course, as the New York Times article points out, Johnson & Johnson
could announce a new bid earlier on Wednesday even though the deadline has
passed, just to draw this drama out even more.
related stories:
Timeline
of
Guidant
Acquisition -- "Voice
in
The
Ear":
The
Stent
Blog
J.&J.
Passes
on
Raising
Guidant
Bid -- New
York
Times
January 24, 2006
Toshiba
and Cardiovascular Institute of the South Establish Cardiovascular
CT Education Partnership
(source: Toshiba America Medical Systems, Inc.)
As multislice CT angiography becomes more widespread, regional centers of excellence
are springing up -- hospitals or imaging centers where physicians can go to
attend training. This press release announces that Toshiba has teamed up with
CIS in south Louisiana to provide training in CT angiography.
 January
23, 2006 January
23, 2006
Boston
Scientific Announces Excellent Results in Study of Patients
with Renal Artery Disease
(source:
Boston
Scientific
Corporation)
The company announced the results of its Renaissance Study at the International
Symposium on Endovascular Therapy (ISET) in Miami Beach. Cardiologist Dr. Krishna
Rocha-Singh of Prairie Cardiovascular Heart Institute in Springfield, IL was
the study's principal investigator. He presented data with show the restenosis
rate in the renal (kidney) arteries was halved through the use of the Express® SD
Renal stent (balloon angioplasty rates are around 40% -- the stent reduced
it to 21.3). Boston Scientific plans to use the data to apply for marketing
approval from the FDA.
January 17, 2006
Guidant
Concludes Boston Scientific Offer is Superior
(source:
Guidant
Corporation)
Minutes before the 5:00pm deadline for Guidant's Board to declare Boston Scientific's
newly increased offer "superior", it has done so, setting in motion
a five-day period for Johnson & Johnson to reply with a new bid, or give
up the acquisition it has worked a year on.
related stories:
Johnson & Johnson Statement Regarding Guidant Corporation Acquisition -- Johnson & Johnson
Disputing Guidant's conclusion that Boston Scientific's offer is superior,
Johnson & Johnson calls the offer "a highly dilutive and leveraged
transaction based on extremely aggressive business projections" and does
not believe the offer will provide shareholders with $80 of value. The press
release also states that Johnson & Johnson will consider its alternatives
under the existing agreement.
Guidant
board deems Boston Scientific bid superior -- Reuters
Guidant
calls
Boston
offer
'superior' -- MarketWatch
Guidant
Backs
$27
Billion
Boston
Scientific
Bid
Over
J&J
Offer -- Bloomberg
News
Timeline
of
Guidant
Acquisition -- "Voice
in
The
Ear":
The
Stent
Blog
January 17, 2006
Guidant
drama revives St. Jude takeover talk
(source: Reuters)
Has someone been reading our Editor's blog?
related stories:
"You've
got to know when to hold 'em, know when to fold 'em" -- "Voice
in The Ear": The Stent Blog (Jan 15)
In summarizing the events of the past week's bidding war, "Voice
in the Ear" posited why St. Jude may be a potential partner for
Boston Scientific, if J&J were to win the Guidant bid
January
17, 2006
Guidant
seeks new offer of $77/shr from J&J
(source:
Reuters)
Reuters is reporting at 3:00pm that Guidant is looking for J&J to offer
$77 per share to counter Boston Scientific's bid of $80 this morning. This
is $1 per share more than J&J was willing to pay a year ago (before Guidant's
defibrillator problems were made public). Evidently the Guidant Board feels
that $3 per share is the differential between the deal going through quickly
(which would be the scenario if Johnson & Johnson acquires them, since
the government regulatory review has already been approved) and a bit of the
unknown if Boston wins out.
January 17, 2006
Boston
Scientific Announces Offer to Acquire Guidant at $80 Per
Share
(source:
Boston
Scientific
Corporation)
Boston Scientific assumed a position this morning that was described by TheStreet.com as "leaning
back for knockout velocity ". The company has raised its bid well beyond
what Wall Street analysts said was needed to beat Johnson & Johnson's offer
from last Friday -- Boston is now offering $9.00/share more. Guidant's Board
has agreed to consider the new offer. They'd better hurry up. Boston Scientific
has placed a 5:00pm deadline today for the Board to declare its offer "superior".
Boston used this same strategy last week when their Thursday night offer included
a 4:00pm Friday deadline. The deadline passed without comment and Guidant and
J&J announced a new higher acquisition agreement later that evening. However,
Boston's new bid is so much higher that Guidant's Board will have significant
difficulty with their shareholders if they do not either accept this offer,
or come up with a new substantially higher agreement with J&J. Some of
the substantial increase in Boston Scientific's new offer is founded on Abbott's
increased participation, higher price for the vascular divisions, larger loan
-- and, new to this agreement -- Abbott will buy 4% of Boston Scientific. More
news at 5!!
related stories:
Boston
Scientific
Raises
Bid
For
Guidant
to
$80
a
Share -- Wall
Street
Journal
$$
Boston
Scientific
hikes
Guidant
offer
again -- Reuters
January
13-14, 2006
Guidant
Corporation and Johnson & Johnson Announce New
Definitive Acquisition Agreement
(source:
Guidant
/
Johnson & Johnson)
Is it finally over?? This was the week that was. The New York Times calls it "the
biggest takeover battle ever in the medical device industry." Guidant
has accepted a newer and higher offer from J&J of $71/share after a day
of ups and downs. Thursday night Boston Scientific made a new and sweeter offer
to Guidant with renewed emphasis on their making concessions so that the FTC
would not have any anti-trust issues -- they even offered to pay in effect
6% interest if the deal took longer than March 31 to close. The new offer came
with a 4:00pm Friday the 13th deadline. During the day Boston briefly extended
the deadline until 6:00pm because, according to reports, Guidant said it was
having logistical difficulties getting everyone together for a meeting by 4:00pm.
But less than a half-hour later, Boston retracted the two hour extension, again
reportedly because it realized that Guidant was using the extra time to renegotiate
a better deal with J&J. Which it did. Guidant has announced that it will
accept the J&J offer, and the Wall Street Journal reports that there were
still anti-trust concerns over the way Boston Scientific was going to divest
parts of Guidant's to Abbott (an issue first raised in last
Monday' s Editor's Blog). It's not entirely clear
what Boston Scientific will now do. They submitted their formal offer to Guidant
last week on a Sunday, so anything could
happen, even over this weekend.
related stories:
"You've
got
to
know
when
to
hold
'em,
know
when
to
fold
'em" -- "Voice
in
The
Ear":
The
Stent
Blog
Guidant
accepts
a
new
J&J
offer -- Wall
Street
Journal
$$
Johnson & Johnson
Pulls
Ahead
in
Takeover
Battle
for
Guidant -- Barnaby
J.
Feder,
New
York
Times
Guidant
accepts
new
J&J
offer
of
$24.2
billion -- Reuters
Guidant
Accepts
Increased
$24.2B
J&J
Bid -- Associated
Press
Guidant
spurns
Boston
Scientific -- Stephen
Heuser,
Boston
Globe
January
12, 2006
Boston
Scientific Improves Offer to Acquire Guidant
(source:
Boston
Scientific)
Sweet sweeter sweetest. If only we had all bought Guidant stock right after
the New York Times ran its series of articles about their defibrillator defect
last spring.... As of tonight, Boston Scientific has increased its bid by $1
a share and guaranteed that the deal will be approved by March 31, or "we'll
pay the interest!!" And if you order now...well actually, that's the case.
Guidant's Board has until 4:00pm Eastern Time tomorrow (Friday) to accept the
offer or it will be withdrawn. If Guidant declares the new deal "superior",
they will have until January 24 to accept it. The question is, if Guidant doesn't
bite, whether Boston Scientific is feeling confident enough to start a proxy
fight for votes at the January 31st stockholders meeting. Several large funds
have criticized Guidant's Board for not accepting Boston's higher offer. Again,
part of the sticking point seems to be the possibility of a longer drawn-out
approval time from the Federal Trade Commission regarding conflicts with product
lines, etc. and anti-trust issues.
related stories:
Boston
Scientific
Sweetens
Deal
for
Guidant -- Wall
Street
Journal
$$
Boston
Scientific
lifts
Guidant
bid -- Stephen
Heuser,
Boston
Globe
Stakes
High
for
Boston
Scientific
in
Bid -- Associated
Press
January
12, 2006
Guidant
Accepts Sweeter J&J Deal
(source: Wall Street Journal $$)
On Wednesday evening, the Guidant Board decided to accept a somewhat raised
offer from Johnson & Johnson, but Boston Scientific says it's not over
till it's over. In a late night press release, Boston states: "Our
discussions with Guidant are ongoing. We intend to vigorously pursue this transaction
to its completion." There is thought that Boston may up its bid as
well. In any case the Board has recommended to the stockholders to approve
the J&J offer. The shareholders will meet on January 31 to vote. Not all
of them agree with the Board, for example the hedge fund Elliott Associates
LP, owner of 3 million Guidant shares, thinks the Boston deal is better. The
Wall Street Journal reports that part of Guidant's concern with the Boston
Scientific deal was "how Boston Scientific would divest itself of Guidant's
stent and catheter businesses." Boston's package included sellling those
to Abbott. The WSJ did not elaborate. We wonder if there were concerns about
the FTC not approving the sale to Abbott, something our Editor discussed in Monday's
blog entry.
related stories:
Boston
Scientific
Comments
on
Johnson & Johnson's
Amended
Agreement
to
Acquire
Guidant -- Boston
Scientific
Guidant
accepts
JJ
offer -- Reuters
Guidant
Accepts
Revised
J&J
Bid
of
$23.2B -- Associated
Press
Won't
You
Be
My
Neighbor? -- "Voice
in
The
Ear":
The
Stent
Blog
January 10, 2006
Guidant's
Board to Meet To Discuss Competing Bids
(source:
Wall
Street
Journal
$$)
3:00pm -- that's when Guidant's Board is scheduled to meet today to decide
between two competing offers for the company: from J&J and Boston Scientific.
There are reports (unconfirmed) that J&J met yesterday with Guidant and
may have upped their bid to counter Boston's bid which was formalized on Sunday.
As of 5:30pm no announcement has been made, but Boston Scientific's stock dropped
4% at triple the normal trading volume. Stay tuned!
related stories:
J&J
may
raise
its
bid
for
Guidant -- Reuters
Johnson
Adds
a
Late
Twist
to
Guidant
Bidding -- New
York
Times
January 10, 2006
Guidant
board to meet on takeover offer
(source:
Reuters)
Guidant's Board is reportedly meeting today as to whether to accept Boston
Scientific's acquisition offer. If it does, J&J would have 5 days to make
a new offer.
related stories:
Elliott
Associates
Believes
Guidant
Bid
by
Boston
Scientific
Superior
to
Johnson & Johnson
Offer -- Elliott
Associates
Hedge
fund
which
is
a
Guidant
shareholder
offered
its
opinion
today
that
anything
lower
than
$71/share
from
J&J
would
not
be
in
the
shareholders'
best
interest
January 8-10,
2006
Boston
Scientific Submits Definitive Offer to Acquire Guidant
for $72 Per Share in Cash and Stock
(source:
Boston
Scientific)
Boston Scientific, after very quickly completing its due diligence, looking
over Guidant's books, has formalized its takeover offer which involves seilling
off Guidant's endovascular business to Abbott for $4.3 billion to encourage
the FTC, which must review the acquisition, that no anti-trust issues will
exist. The details were discussed in an analyst conference call. Boston Scientific
has added a "collar" to the deal, so that if its stock value falls,
the conversion ratio for Guidant shareholders will be upped. Guidant's board
now has to decide whether to accept the bid. J&J who made the initial bid
and then lowered it, due to Guidant's legal problems from fault ICDs, will
have 5 days to rebid. Meanwhile Guidant shareholders will be voting on the
J&J bid at the end of January. This is a very complicated and high stakes
situation for all companies involved, although Abbott stands to gain no matter
which way the decision goes.
related stories:
Boston
Scientific
formalizes
$25
Billion
Guidant
bid -- Reuters
Boston
Scientific
looks
to
Guidant
to
boost
sales -- Reuters
Quick
Thoughts
on
Boston/Guidant
Deal -- "Voice
in
The
Ear":
The
Stent
Blog
Boston
Scientific
Formalizes
Bid
To
Buy
Guidant -- Wall
Street
Journal
$$
Boston
Scientific
Now
Says
Buying
Guidant
Would
Not
Increase
Profits
Until
'09 -- New
York
Times
Times
reporter
Barnaby
J.
Feder
reports
in
depth
on
some
cautions
in
the
Boston
Scientific/Guidant
merger
from
the
Guidant
side
that
have
caused
Boston
Scientific
to extend the
period
that
it
may
take
to
make
the
acquisition
profitable.
Boston
Scientific
Pushes
Back
Expectations -- Mark
Jewell,
Associated
Press
January 6, 2006
Boston
Scientific Expects Imminent Guidant Agreement; CEO to
Stay
(source:
Mark
Jewell,
AP
Business
Writer)
Boston Scientific expects to finalize its offer to buy Guidant by the end of
next week. Guidant shareholders are scheduled to vote on the smaller and earlier
offer by Johnson & Johnson on January 31. Boston also announced that Jim
Tobin would be staying on as CEO and president "for the foreseeable future." There
had been speculation that he might be leaving -- in a recent analysts' meeting
he had stated that he was continuing to hand over more and more of his responsibilities
to COO Paul LaViolette, to free him up to work on other things. Wall Street
analysts have said the stability of Tobin remaining should be comforting to
Guidant shareholders.
related stories:
Tobin
to
stay
at
Boston
Scientific -- Stephen
Heuser,
Boston
Globe
Tobin
won’t
abridge
BSX
tenure -- Jennifer
Heldt
Powell,
Boston
Herald
2nd
suitor
preparing
bid
to
buy
Guidant --
Jeff
Swiatek,
Indianapolis
Star
January
3, 2006
Coronary
CT workups save time, money
(source:
In-Sung
Yoo,
Delaware
Online)
Is cardiac CT scanning an alternative to cardiac catheterization for screening
patients? This article from a local Delaware paper speaks to some physicians
about this growing imaging technology, known as MultiSlice Computed Tomography
or MSCT.
related stories:
New
scan
gives
doctors
better
picture
of
heart -- WBIR-TV,
Knoxville
TN
(December
5, 2005) |
| Click
here for more information about the following ads
|
|
